Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia
- PMID: 20124115
- PMCID: PMC4208310
- DOI: 10.1001/archgenpsychiatry.2009.196
Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia
Abstract
Context: Chondroitin sulfate proteoglycans (CSPGs), a main component of the brain extracellular matrix, regulate developmental and adult neural functions that are highly relevant to the pathogenesis of schizophrenia. Such functions, together with marked expression of CSPGs in astrocytes within the normal human amygdala and evidence of a disruption of astrocytic functions in this disease, point to involvement of CSPG-glial interactions in schizophrenia.
Hypothesis: Chondroitin sulfate proteoglycan-related abnormalities involve glial cells and extracellular matrix pericellular aggregates (perineuronal nets) in the amygdala and entorhinal cortex of subjects with schizophrenia.
Design: Postmortem case-control study.
Setting: The Translational Neuroscience Laboratory at McLean Hospital, Harvard Medical School. Specimens were obtained from the Harvard Brain Tissue Resource Center at McLean Hospital.
Participants: Two separate cohorts of healthy control (n = 15; n = 10) and schizophrenic (n = 11; n = 10) subjects and a cohort of subjects with bipolar disorder (n = 11).
Interventions: Quantitative, immunocytological, and histological postmortem investigations.
Main outcome measures: Numerical densities of CSPG-positive glial cells and perineuronal nets, glial fibrillary acidic protein-positive astrocytes, and total numbers of parvalbumin-positive neurons in the deep amygdala nuclei and entorhinal cortex.
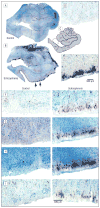
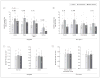
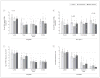
Results: In schizophrenia, massive increases in CSPG-positive glial cells were detected in the deep amygdala nuclei (419%-1162%) and entorhinal cortex (layer II; 480%-1560%). Perineuronal nets were reduced in the lateral nucleus of the amygdala and lateral entorhinal cortex (layer II). Numerical densities of glial fibrillary acidic protein-positive glial cells and total numbers of parvalbumin-positive neurons were unaltered. Changes in CSPG-positive elements were negligible in subjects with bipolar disorder.
Conclusions: Marked changes in functionally relevant molecules in schizophrenia point to a pivotal role for extracellular matrix-glial interactions in the pathogenesis of this disease. Disruption of these interactions, unsuspected thus far, may represent a unifying factor contributing to disturbances of neuronal migration, synaptic connectivity, and GABAergic, glutamatergic, and dopaminergic neurotransmission in schizophrenia. The lack of CSPG abnormalities in bipolar disorder points to a distinctive aspect of the pathophysiology of schizophrenia in key medial temporal lobe regions.
Figures

Similar articles
-
Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala.Transl Psychiatry. 2015 Jan 20;5(1):e496. doi: 10.1038/tp.2014.128. Transl Psychiatry. 2015. PMID: 25603412 Free PMC article.
-
Extracellular matrix abnormalities in schizophrenia.Neuropharmacology. 2012 Mar;62(3):1584-97. doi: 10.1016/j.neuropharm.2011.08.010. Epub 2011 Aug 16. Neuropharmacology. 2012. PMID: 21856318 Free PMC article. Review.
-
Total number, distribution, and phenotype of cells expressing chondroitin sulfate proteoglycans in the normal human amygdala.Brain Res. 2008 May 1;1207:84-95. doi: 10.1016/j.brainres.2008.02.036. Epub 2008 Mar 4. Brain Res. 2008. PMID: 18374308 Free PMC article.
-
Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia.Schizophr Res. 2013 Nov;150(2-3):366-72. doi: 10.1016/j.schres.2013.08.013. Epub 2013 Sep 10. Schizophr Res. 2013. PMID: 24035561 Free PMC article.
-
The tetrapartite synapse: a key concept in the pathophysiology of schizophrenia.Eur Psychiatry. 2018 Apr;50:60-69. doi: 10.1016/j.eurpsy.2018.02.003. Epub 2018 Mar 2. Eur Psychiatry. 2018. PMID: 29503098 Free PMC article. Review.
Cited by
-
Semaphorins in Adult Nervous System Plasticity and Disease.Front Synaptic Neurosci. 2021 May 11;13:672891. doi: 10.3389/fnsyn.2021.672891. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34045951 Free PMC article. Review.
-
A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia.Transl Psychiatry. 2013 Jan 15;3(1):e215. doi: 10.1038/tp.2012.145. Transl Psychiatry. 2013. PMID: 23321812 Free PMC article.
-
Molecular signature of extracellular matrix pathology in schizophrenia.Eur J Neurosci. 2021 Jun;53(12):3960-3987. doi: 10.1111/ejn.15009. Epub 2020 Nov 13. Eur J Neurosci. 2021. PMID: 33070392 Free PMC article.
-
MiR-29 coordinates age-dependent plasticity brakes in the adult visual cortex.EMBO Rep. 2020 Nov 5;21(11):e50431. doi: 10.15252/embr.202050431. Epub 2020 Oct 7. EMBO Rep. 2020. PMID: 33026181 Free PMC article.
-
Cell Type-Specific Effects of Mutant DISC1: A Proteomics Study.Mol Neuropsychiatry. 2016 May;2(1):28-36. doi: 10.1159/000444587. Epub 2016 Apr 1. Mol Neuropsychiatry. 2016. PMID: 27606318 Free PMC article.
References
-
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–253. - PubMed
-
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. - PubMed
-
- Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50(2):291–301. - PubMed
-
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13(2):54–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

