Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients
- PMID: 20124171
- PMCID: PMC4872305
- DOI: 10.1200/JCO.2009.25.7246
Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients
Abstract
Purpose: The clinical efficacy of tamoxifen is suspected to be influenced by the activity of drug-metabolizing enzymes and transporters involved in the formation, metabolism, and elimination of its active forms. We investigated relationships of polymorphisms in transporter genes and CYP2D6 to clinical outcome of patients receiving tamoxifen.
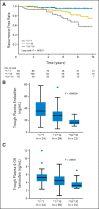
Patients and methods: We studied 282 patients with hormone receptor-positive, invasive breast cancer receiving tamoxifen monotherapy, including 67 patients who have been previously reported. We investigated the effects of allelic variants of CYP2D6 and haplotype-tagging single nucleotide polymorphisms (tag-SNPs) of ABCB1, ABCC2, and ABCG2 on recurrence-free survival using the Kaplan-Meier method and Cox regression analysis. Plasma concentrations of tamoxifen metabolites were measured in 98 patients receiving tamoxifen 20 mg/d.
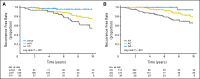
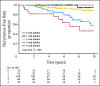
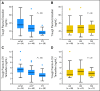
Results: CYP2D6 variants were significantly associated with shorter recurrence-free survival (P = .000036; hazard ratio [HR] = 9.52; 95% CI, 2.79 to 32.45 in patients with two variant alleles v patients without variant alleles). Among 51 tag-SNPs in transporter genes, a significant association was found at rs3740065 in ABCC2 (P = .00017; HR = 10.64; 95% CI, 1.44 to 78.88 in patients with AA v GG genotypes). The number of risk alleles of CYP2D6 and ABCC2 showed cumulative effects on recurrence-free survival (P = .000000055). Patients carrying four risk alleles had 45.25-fold higher risk compared with patients with <or= one risk allele. CYP2D6 variants were associated with lower plasma levels of endoxifen and 4-hydroxytamoxifen (P = .0000043 and .00052), whereas no significant difference was found among ABCC2 genotype groups.
Conclusion: Our results suggest that polymorphisms in CYP2D6 and ABCC2 are important predictors for the prognosis of patients with breast cancer treated with tamoxifen.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Evidence and practice regarding the role for CYP2D6 inhibition in decisions about tamoxifen therapy.J Clin Oncol. 2010 Mar 10;28(8):1273-5. doi: 10.1200/JCO.2009.26.7906. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124162 No abstract available.
-
ABCC2 and clinical outcome of tamoxifen therapy.J Clin Oncol. 2010 Sep 1;28(25):e448; author reply e449. doi: 10.1200/JCO.2010.29.6665. Epub 2010 Jul 12. J Clin Oncol. 2010. PMID: 20625134 No abstract available.
References
-
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. - PubMed
-
- Borgna JL, Rochefort H. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem. 1981;256:859–868. - PubMed
-
- Lien EA, Solheim E, Lea OA, et al. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49:2175–2183. - PubMed
-
- Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

