Cytokine working group study of lymphodepleting chemotherapy, interleukin-2, and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma: clinical outcomes and peripheral-blood cell recovery
- PMID: 20124177
- PMCID: PMC2834469
- DOI: 10.1200/JCO.2009.24.8153
Cytokine working group study of lymphodepleting chemotherapy, interleukin-2, and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma: clinical outcomes and peripheral-blood cell recovery
Abstract
Purpose: Recovery of lymphocyte populations after lymphocyte depletion is implicated in therapeutic immune pathways in animal models and in patients with cancer. We sought to evaluate the effects of chemotherapy-induced lymphodepletion followed by granulocyte-macrophage colony-stimulating factor (GM-CSF) and high-dose interleukin-2 (IL-2) therapy on clinical response and the recovery of lymphocyte subcompartments in patients with metastatic melanoma.
Patients and methods: This was a two-stage phase II trial design. Patients with measurable metastatic melanoma were treated with intravenous cyclophosphamide (60 mg/kg, days 1 and 2) and fludarabine (25 mg/m(2), day 3 through 7) followed by two 5-day courses of intravenous high-dose bolus IL-2 (600,000 U/kg; days 8 through 12 and 21 through 25). GM-CSF (250 microg/m(2)/d beginning day 8) was given until granulocyte recovery. Lymphocyte recovery profiles were determined by flow cytometric phenotyping at regular intervals, and clinical outcome was assessed by Response Evaluation Criteria in Solid Tumors (RECIST).
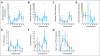
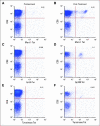
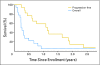
Results: The trial was stopped at the end of stage 1 with four of 18 objective responses noted. Twelve patients had detailed lymphocyte subcompartments evaluated. After lymphodepletion, we observed an induction of regulatory cells (CD4+ T regulatory cells; CD8+ T suppressor cells) and of T memory cells (CD8+ T central memory cells; T effector memory RA+ cells). Expansion of circulating melanoma-specific CD8(+) cells was observed in one of four HLA-A2-positive patients.
Conclusion: Chemotherapy-induced lymphodepletion modulates the homeostatic repopulation of the lymphocyte compartment and influences recovering lymphocyte subpopulations. Clinical activity seems similar to standard high-dose aldesleukin alone.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Phase I trial of combined immunotherapy with subcutaneous granulocyte macrophage colony-stimulating factor, low-dose interleukin 2, and interferon alpha in progressive metastatic melanoma and renal cell carcinoma.Clin Cancer Res. 2000 Apr;6(4):1267-72. Clin Cancer Res. 2000. PMID: 10778950 Clinical Trial.
-
Immune effects of escalating doses of granulocyte-macrophage colony-stimulating factor added to a fixed, low-dose, inpatient interleukin-2 regimen: a randomized phase I trial in patients with metastatic melanoma and renal cell carcinoma.J Immunother. 2003 Mar-Apr;26(2):130-8. doi: 10.1097/00002371-200303000-00005. J Immunother. 2003. PMID: 12616104 Clinical Trial.
-
Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells.J Clin Oncol. 2003 Nov 1;21(21):4016-26. doi: 10.1200/JCO.2003.10.005. J Clin Oncol. 2003. PMID: 14581425 Clinical Trial.
-
Granulocyte/macrophage-colony-stimulating-factor plus interleukin-2 plus interferon alpha in the treatment of metastatic renal cell carcinoma: a pilot study.Cancer Immunol Immunother. 2001 Jan;49(11):613-20. doi: 10.1007/s002620000159. Cancer Immunol Immunother. 2001. PMID: 11225992 Free PMC article. Clinical Trial.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
What is the role of chemotherapy in the treatment of melanoma?Curr Treat Options Oncol. 2014 Jun;15(2):321-35. doi: 10.1007/s11864-014-0277-5. Curr Treat Options Oncol. 2014. PMID: 24599525 Review.
-
Role of high-dose interleukin-2 for melanoma in the age of cellular therapy.J Immunother Cancer. 2025 May 30;13(5):e011119. doi: 10.1136/jitc-2024-011119. J Immunother Cancer. 2025. PMID: 40447314 Free PMC article. Review.
-
Immunomodulatory cytokines as therapeutic agents for melanoma.Immunotherapy. 2011 May;3(5):673-90. doi: 10.2217/imt.11.45. Immunotherapy. 2011. PMID: 21554095 Free PMC article. Review.
-
The tumor microenvironment expression of p-STAT3 influences the efficacy of cyclophosphamide with WP1066 in murine melanoma models.Int J Cancer. 2012 Jul 1;131(1):8-17. doi: 10.1002/ijc.26307. Epub 2011 Aug 24. Int J Cancer. 2012. PMID: 21792892 Free PMC article.
-
Lymphodepletion - an essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle.Front Immunol. 2023 Dec 22;14:1303935. doi: 10.3389/fimmu.2023.1303935. eCollection 2023. Front Immunol. 2023. PMID: 38187393 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. - PubMed
-
- Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

