Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients
- PMID: 20124181
- PMCID: PMC2834472
- DOI: 10.1200/JCO.2009.24.2099
Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients
Abstract
Purpose: The Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) project aims to additionally explore the data of the two large, randomized, cooperative-group studies comparing two doses of imatinib (400 mg daily v twice daily) in 1,640 patients with advanced GIST.
Methods: End points were progression-free survival (PFS) and overall survival (OS). Investigated cofactors included age, sex, performance status (PS), primary tumor site, time from diagnosis, prior therapies, baseline biology, and KIT/PDGFRalpha mutations for a subset of 772 patients. Univariate and multivariate models were used for the analysis.
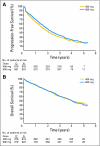
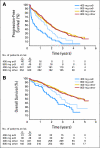
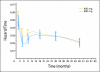
Results: At a median follow-up of 45 months, a small but significant PFS advantage was documented for the high-dose arm. OS was identical in the two arms. The multivariate prognostic models included the following adverse factors: male sex, poor PS, and high baseline neutrophils counts (PFS and OS); low hemoglobin and GIST from small bowel origin (PFS); and advanced age, large tumor size, low albumin level, and prior chemotherapy (OS). In patients analyzed for mutations, patients with wild type, patients with KIT exon 9 mutations, and patients with other mutations had worse prognoses than patients with KIT exon 11 mutations for both end points. The mutation status was the only predictive factor for the PFS benefit attributed to high-dose treatment that resulted in significantly longer PFS (and higher objective response rate) for patients with KIT exon 9 mutations.
Conclusion: This analysis confirms a small PFS advantage of high-dose imatinib, essentially among patients with KIT exon 9 mutations, but no OS advantage.
Conflict of interest statement
Disclosures of potential conflicts of interest and author contributions for the MetaGIST Group writing committee are found at the end of this article.
Figures

References
-
- Nilsson B, Bümming P, Meis-Kindblom J, et al. Gastrointestinal stomal tumors: The incidence, prevalence, clinical course and prognostication in the preimatinib era—A population based study in western Sweden. Cancer. 2005;103:821–829. - PubMed
-
- Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–664. - PubMed
-
- van Oosterom AT, Judson I, Verweij J, et al. For the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group: Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours—A phase I study. Lancet. 2001;358:1421–1423. - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. - PubMed
-
- Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target: Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006–2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- R01 CA106588/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA086780/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- U10 CA027525/CA/NCI NIH HHS/United States
- U10 CA035091/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- U10 CA071323/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- U10 CA063845/CA/NCI NIH HHS/United States
- U10 CA063850/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- U10 CA011488/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA114558/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA035262/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- U10 CA003927/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- U10 CA068183/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

