Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy
- PMID: 20124182
- PMCID: PMC4979216
- DOI: 10.1200/JCO.2009.24.2024
Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy
Abstract
PURPOSE; Pertuzumab, a human epidermal growth factor receptor 2 (HER2) -targeted monoclonal antibody, potently inhibits HER2 dimerization and HER-mediated signaling pathways. Pertuzumab and the approved HER2-targeted monoclonal antibody trastuzumab have complementary mechanisms of action and result in enhanced antitumor activity when combined. This phase II trial assessed the efficacy and safety profile of the combination in patients with HER2-positive breast cancer whose disease had progressed during prior trastuzumab-based therapy.
Patients and methods: This was a multicenter, open-label, single-arm, Simon two-stage study. Patients with advanced HER2-positive breast cancer in whom disease progression had occurred during prior trastuzumab-based therapy received trastuzumab weekly (4 mg/kg loading dose, then 2 mg/kg every week) or every 3 weeks (8 mg/kg loading dose, then 6 mg/kg every 3 weeks) and pertuzumab every 3 weeks (840 mg loading dose, then 420 mg every 3 weeks). Treatment continued until disease progression or excessive toxicity.
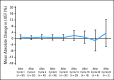
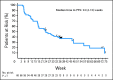
Results: All 66 patients were assessable for efficacy and safety. The objective response rate was 24.2%, and the clinical benefit rate was 50%. Five patients (7.6%) experienced a complete response, 11 patients (16.7%) experienced a partial response, and 17 patients (25.8%) experienced stable disease of > or = 6 months. Median progression-free survival was 5.5 months. Overall, the combination of pertuzumab and trastuzumab was well tolerated, and adverse events were mild to moderate. Cardiac dysfunction was minimal, and no patients withdrew as a result of cardiac-related adverse events.
Conclusion: The combination of pertuzumab and trastuzumab is active and well tolerated in patients with metastatic HER2-positive breast cancer who had experienced progression during prior trastuzumab therapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Optimizing the delivery of targeted research: an opportunity for comparative effectiveness research.J Clin Oncol. 2010 Mar 1;28(7):1089-91. doi: 10.1200/JCO.2009.25.2205. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124174 No abstract available.
-
Whither HER2-related therapeutics?J Clin Oncol. 2010 Mar 1;28(7):1091-6. doi: 10.1200/JCO.2009.25.8624. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124168 No abstract available.
References
-
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Smith IE. Efficacy and safety of Herceptin in women with metastatic breast cancer: Results from pivotal clinical studies. Anticancer Drugs. 2001;12(suppl 4):S3–S10. - PubMed
-
- Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. J Clin Oncol. 2007;25(suppl):6s. abstr 512.
-
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous