Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer
- PMID: 20124183
- PMCID: PMC4979215
- DOI: 10.1200/JCO.2009.24.1661
Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer
Abstract
Purpose: Pertuzumab is a humanized monoclonal antibody inhibiting human epidermal growth factor receptor 2 (HER2) dimerization. The aim of this phase II trial was to assess the antitumor activity and safety profile of pertuzumab monotherapy in patients with HER2-negative metastatic breast cancer. The utility of biomarkers detected in paraffin-embedded tissue as predictors of response was also explored.
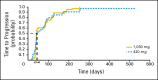
Patients and methods: This was an international, multicenter, open-label, randomized phase II study. Patients (n = 79) with centrally confirmed HER2-negative metastatic breast cancer were randomly assigned to receive pertuzumab once every 3 weeks with a loading dose of 840 mg followed thereafter by either 420 mg (arm A) or 1,050 mg (arm B). Patients were stratified by country and prior taxane therapy.
Results: Of 79 patients who were randomly assigned, 78 were included in the intent-to-treat population. In arm A (n = 41), two patients had partial responses, and 18 patients (44%) experienced stable disease (SD) lasting > or = 12 weeks. In arm B (n = 37), SD was observed in 14 patients (38%). Overall, six of 78 patients responded or had SD > or = 6 months. Pertuzumab was generally well tolerated, and most adverse events were mild to moderate. Decline in left ventricular ejection fraction of > or = 10% and/or to less than 50% was observed in eight patients, with one case of congestive heart failure in arm A. Pharmacokinetic data supported a fixed dose of pertuzumab once every 3 weeks.
Conclusion: The limited efficacy observed in this study, generally SD of relatively short duration, suggested little benefit of further investigation of single-agent pertuzumab in unselected patients with HER2-negative disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Optimizing the delivery of targeted research: an opportunity for comparative effectiveness research.J Clin Oncol. 2010 Mar 1;28(7):1089-91. doi: 10.1200/JCO.2009.25.2205. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124174 No abstract available.
-
Whither HER2-related therapeutics?J Clin Oncol. 2010 Mar 1;28(7):1091-6. doi: 10.1200/JCO.2009.25.8624. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124168 No abstract available.
Similar articles
-
Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy.J Clin Oncol. 2010 Mar 1;28(7):1138-44. doi: 10.1200/JCO.2009.24.2024. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124182 Free PMC article. Clinical Trial.
-
Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group.Lancet Oncol. 2018 Mar;19(3):323-336. doi: 10.1016/S1470-2045(18)30083-4. Epub 2018 Feb 9. Lancet Oncol. 2018. PMID: 29433963 Clinical Trial.
-
Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study.Lancet Oncol. 2021 Jan;22(1):85-97. doi: 10.1016/S1470-2045(20)30536-2. Epub 2020 Dec 21. Lancet Oncol. 2021. PMID: 33357420 Clinical Trial.
-
Pertuzumab for the treatment of human epidermal growth factor receptor type 2-positive metastatic breast cancer.Am J Health Syst Pharm. 2013 Sep 15;70(18):1579-87. doi: 10.2146/ajhp120735. Am J Health Syst Pharm. 2013. PMID: 23988598 Review.
-
HER-dimerization inhibitors: evaluating pertuzumab in women's cancers.Expert Opin Biol Ther. 2010 Feb;10(2):243-50. doi: 10.1517/14712590903514090. Expert Opin Biol Ther. 2010. PMID: 20001562 Review.
Cited by
-
Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates.Ann Transl Med. 2014 Dec;2(12):122. doi: 10.3978/j.issn.2305-5839.2014.08.13. Ann Transl Med. 2014. PMID: 25568875 Free PMC article. Review.
-
Consensus on clinical diagnosis and medical treatment of HER2-low breast cancer (2022 edition).J Natl Cancer Cent. 2023 Sep 29;3(4):266-272. doi: 10.1016/j.jncc.2023.09.002. eCollection 2023 Dec. J Natl Cancer Cent. 2023. PMID: 39036662 Free PMC article.
-
HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer.Front Mol Biosci. 2022 Mar 15;9:834651. doi: 10.3389/fmolb.2022.834651. eCollection 2022. Front Mol Biosci. 2022. PMID: 35372498 Free PMC article. Review.
-
Treatment of HER2-positive breast cancer.Breast. 2014 Apr;23(2):128-136. doi: 10.1016/j.breast.2013.11.011. Epub 2013 Dec 19. Breast. 2014. PMID: 24360619 Free PMC article. Review.
-
Newer therapies for the treatment of metastatic breast cancer: a clinical update.Indian J Pharm Sci. 2013 May;75(3):251-61. doi: 10.4103/0250-474X.117396. Indian J Pharm Sci. 2013. PMID: 24082340 Free PMC article. Review.
References
-
- Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–130. - PubMed
-
- Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous