Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma
- PMID: 20124186
- PMCID: PMC2834468
- DOI: 10.1200/JCO.2009.23.2595
Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma
Abstract
Purpose: This phase III open-label study compared the efficacy and safety of enzastaurin versus lomustine in patients with recurrent glioblastoma (WHO grade 4).
Patients and methods: Patients were randomly assigned 2:1 to receive 6-week cycles of enzastaurin 500 mg/d (1,125-mg loading dose, day 1) or lomustine (100 to 130 mg/m(2), day 1). Assuming a 45% improvement in progression-free survival (PFS), 397 patients were required to provide 80% power to achieve statistical significance at a one-sided level of .025.
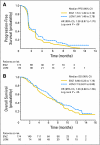
Results: Enrollment was terminated at 266 patients (enzastaurin, n = 174; lomustine, n = 92) after a planned interim analysis for futility. Patient characteristics were balanced between arms. Median PFS (1.5 v 1.6 months; hazard ratio [HR] = 1.28; 95% CI, 0.97 to 1.70), overall survival (6.6 v 7.1 months; HR = 1.20; 95% CI, 0.88 to 1.65), and 6-month PFS rate (P = .13) did not differ significantly between enzastaurin and lomustine, respectively. Stable disease occurred in 38.5% and 35.9% of patients and objective response occurred in 2.9% and 4.3% of patients, respectively. Time to deterioration of physical and functional well-being and symptoms did not differ between arms (HR = 1.12; P = .54). Four patients discontinued enzastaurin because of drug-related serious adverse events (AEs). Eleven patients treated with enzastaurin died on study (four because of AEs; one was drug-related). All four deaths that occurred in patients receiving lomustine were disease-related. Grade 3 to 4 hematologic toxicities were significantly higher with lomustine (46 events) than with enzastaurin (one event; P < or = .001).
Conclusion: Enzastaurin was well tolerated and had a better hematologic toxicity profile but did not have superior efficacy compared with lomustine in patients with recurrent glioblastoma.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Enzastaurin in the treatment of recurrent glioblastoma: a promise that did not materialize.J Clin Oncol. 2010 Mar 1;28(7):1097-8. doi: 10.1200/JCO.2009.26.1149. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124164 No abstract available.
References
-
- Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet. 2003;361:323–331. - PubMed
-
- DeAngelis LM. Chemotherapy for brain tumors: A new beginning. N Engl J Med. 2005;352:1036–1038. - PubMed
-
- Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin Oncol. 2004;31:635–644. - PubMed
-
- Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1–9. - PubMed
-
- Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

