Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial
- PMID: 20124207
- PMCID: PMC2816006
- DOI: 10.1212/WNL.0b013e3181ccc6ef
Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial
Abstract
Objective: Pregabalin is effective in several neuropathic pain syndromes. This trial evaluated its efficacy, safety, and tolerability for treatment of painful HIV-associated neuropathy.
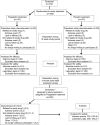
Methods: This randomized, double-blind, placebo-controlled, parallel-group trial included a 2-week double-blind dose-adjustment (150-600 mg/day BID) phase, a 12-week double-blind maintenance phase, and an optional 3-month open label extension phase. The primary efficacy measure was the mean Numeric Pain Rating Scale (NPRS) score, an 11-point numeric rating scale. Secondary measures included Patient Global Impression of Change (PGIC) and sleep measurements.
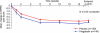
Results: Baseline mean NPRS score was 6.93 for patients randomized to pregabalin (n = 151) and 6.72 for those to placebo (n = 151). Pregabalin average daily dosage (SD) was 385.7 (160.3) mg/d. At endpoint, pregabalin and placebo showed substantial reductions in mean NPRS score from baseline: -2.88 vs -2.63, p = 0.3941. Pregabalin had greater improvements in NPRS score relative to placebo at weeks 1 (-1.14 vs -0.69, p = 0.0131) and 2 (-1.92 vs -1.43, p = 0.0393), and at weeks 7 (-3.22 vs -2.53 p = 0.0307) and 8 (-3.33 vs -2.53, p = 0.0156). At all other time points, differences between groups were not significant. Sleep measurements and 7-item PGIC did not differ among treatment groups; however, collapsed PGIC scores showed 82.8% of pregabalin and 66.7% of placebo patients rated themselves in 1 of the 3 "improved" categories (p = 0.0077). Somnolence and dizziness were the most common adverse events with pregabalin.
Conclusions: Pregabalin was well-tolerated, but not superior to placebo in the treatment of painful HIV neuropathy. Factors predicting analgesic response in HIV neuropathy warrant additional research.
Classification of evidence: This Class II trial showed that pregabalin is not more effective than placebo in treatment of painful HIV neuropathy.
Figures
References
-
- Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: The Manhattan HIV Brain Bank. Arch Neurol 2004;61:546–551. - PubMed
-
- Simpson DM, Kitch D, Evans SR, et al, ACTG A5117 Study Group. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology 2006;66:1679–1687. - PubMed
-
- Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol 1995;9:153–161. - PubMed
-
- Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. AIDS 2002;16:2105–2117. - PubMed
-
- Verma A. Epidemiology and clinical features of HIV-1 associated neuropathies. J Periph Nerv Syst 2001;6:8–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 3857-53187/PHS HHS/United States
- R01 DK063296/DK/NIDDK NIH HHS/United States
- MH 22005/MH/NIMH NIH HHS/United States
- R01 NS046710/NS/NINDS NIH HHS/United States
- R24 MH59724/MH/NIMH NIH HHS/United States
- R01-NS-328-05/NS/NINDS NIH HHS/United States
- MH058076/MH/NIMH NIH HHS/United States
- R03 DA022137/DA/NIDA NIH HHS/United States
- UO1 AI69495/AI/NIAID NIH HHS/United States
- R01-NS-41198/NS/NINDS NIH HHS/United States
- P01-MH64409/MH/NIMH NIH HHS/United States
- U01-A1069511/PHS HHS/United States
- UO1 NS32228/NS/NINDS NIH HHS/United States
- R01-NS036524/NS/NINDS NIH HHS/United States
- R01 HL059459/HL/NHLBI NIH HHS/United States
- P01-MH064570/MH/NIMH NIH HHS/United States
- 00-AI-0005/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials