Differential expression of claudin tight junction proteins in the human cortical nephron
- PMID: 20124215
- PMCID: PMC2891746
- DOI: 10.1093/ndt/gfq006
Differential expression of claudin tight junction proteins in the human cortical nephron
Abstract
Background: In renal tubules, paracellular permeability is tightly controlled to facilitate solute absorption and urinary concentration and is regulated by tight junctions, which incorporate claudin proteins. There is very limited information confirming the localization of these proteins in the human renal cortex. Most data is inferred from mouse, bovine and rabbit studies and differences exist between mouse and other species.
Methods: A survey of claudin staining was performed on human kidney cortex embedded in glycolmethacrylate resin to enhance tissue morphology and facilitate the cutting of 2 microm serial sections.
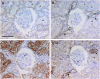
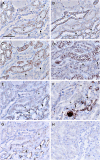
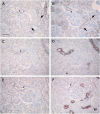
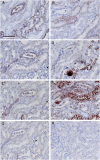
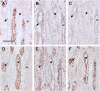
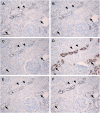
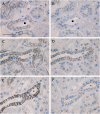
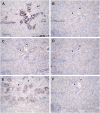
Results: Claudin-2, -10 and -11 antibodies labelled renal tubular epithelial cells, correlating with Lotus tetragonolobus and N-cadherin positive proximal tubules. Claudin-3, -10, -11 and -16 antibodies strongly stained a population of tubules that were positive for Tamm Horsfall protein on adjacent sections, confirming expression in the thick ascending limb of the Loop of Henle. Claudin-3, -4 and -8 antibodies reacted with tubules that correlated with the distal nephron markers, E-cadherin, epithelial membrane antigen and Dolichos biflorus and claudin-3, -4, -7 and -8 with the distal tubule marker, calbindin, and the collecting duct marker, aquaporin-2. Claudin-14 was localized in distal convoluted tubules, correlating positively with calbindin but negatively with aquaporin-2, whereas claudin-1 staining was identified in the parietal epithelium of Bowman's capsule, distal convoluted tubule and collecting duct. Cellular and tight junction localization of claudin staining in renal tubules was heterogeneous and is discussed.
Conclusions: Complex variation in the expression of human claudins likely determines paracellular permeability in the kidney. Altered claudin expression may influence pathologies involving abnormalities of absorption.
Figures
Similar articles
-
Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments.J Am Soc Nephrol. 2002 Apr;13(4):875-886. doi: 10.1681/ASN.V134875. J Am Soc Nephrol. 2002. PMID: 11912246
-
Restricted localization of claudin-16 at the tight junction in the thick ascending limb of Henle's loop together with claudins 3, 4, and 10 in bovine nephrons.J Vet Med Sci. 2006 May;68(5):453-63. doi: 10.1292/jvms.68.453. J Vet Med Sci. 2006. PMID: 16757888
-
Expression of claudin-7 and -8 along the mouse nephron.Am J Physiol Renal Physiol. 2004 Jun;286(6):F1063-71. doi: 10.1152/ajprenal.00384.2003. Epub 2004 Jan 13. Am J Physiol Renal Physiol. 2004. PMID: 14722018
-
Physiological roles of claudins in kidney tubule paracellular transport.Am J Physiol Renal Physiol. 2017 Jan 1;312(1):F9-F24. doi: 10.1152/ajprenal.00204.2016. Epub 2016 Oct 26. Am J Physiol Renal Physiol. 2017. PMID: 27784693 Review.
-
Claudins in barrier and transport function-the kidney.Pflugers Arch. 2017 Jan;469(1):105-113. doi: 10.1007/s00424-016-1906-6. Epub 2016 Nov 23. Pflugers Arch. 2017. PMID: 27878608 Free PMC article. Review.
Cited by
-
Endotoxemia alters tight junction gene and protein expression in the kidney.Am J Physiol Renal Physiol. 2012 Sep 15;303(6):F821-30. doi: 10.1152/ajprenal.00023.2012. Epub 2012 Jul 11. Am J Physiol Renal Physiol. 2012. PMID: 22791339 Free PMC article.
-
Precision Medicine Diagnostics for Rare Kidney Disease: Twitter as a Tool in Clinical Genomic Translation.Kidney Med. 2019 Aug 14;1(5):315-318. doi: 10.1016/j.xkme.2019.06.006. eCollection 2019 Sep-Oct. Kidney Med. 2019. PMID: 32734212 Free PMC article.
-
Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction.PLoS One. 2014 Jan 15;9(1):e85775. doi: 10.1371/journal.pone.0085775. eCollection 2014. PLoS One. 2014. PMID: 24454932 Free PMC article.
-
Claudins in Renal Physiology and Pathology.Genes (Basel). 2020 Mar 10;11(3):290. doi: 10.3390/genes11030290. Genes (Basel). 2020. PMID: 32164158 Free PMC article. Review.
-
One gene, two paracellular ion channels-claudin-10 in the kidney.Pflugers Arch. 2017 Jan;469(1):115-121. doi: 10.1007/s00424-016-1921-7. Epub 2016 Dec 10. Pflugers Arch. 2017. PMID: 27942952 Review.
References
-
- Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F461–F471. - PubMed
-
- Berry CA, Rector FC., Jr Mechanism of proximal NaCl reabsorption in the proximal tubule of the mammalian kidney. Semin Nephrol. 1991;11:86–97. - PubMed
-
- Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol. 1997;273:F179–F192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

