Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes
- PMID: 20124223
- PMCID: PMC2877972
- DOI: 10.1074/mcp.M900381-MCP200
Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes
Abstract
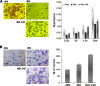
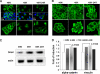
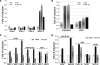
Under hypoxia, tumor cells produce a secretion that modulates their microenvironment to facilitate tumor angiogenesis and metastasis. Here, we observed that hypoxic or reoxygenated A431 carcinoma cells exhibited enhanced angiogenic and metastatic potential such as reduced cell-cell and cell-extracellular matrix adhesion, increased invasiveness, and production of a secretion with increased chorioallantoic membrane angiogenic activity. Consistent with these observations, quantitative proteomics revealed that under hypoxia the tumor cells secreted proteins involved in angiogenesis, focal adhesion, extracellular matrix-receptor interaction, and immune cell recruitment. Unexpectedly, the secreted proteins were predominantly cytoplasmic and membrane proteins. Ultracentrifugation at 100,000 x g precipitated 54% of the secreted proteins and enriched for many exosome-associated proteins such as the tetraspanins and Alix and also proteins with the potential to facilitate angiogenesis and metastasis. Two tetraspanins, CD9 and CD81, co-immunoprecipitated. Together, these data suggested that tumor cells secrete proteins and exosomes with the potential to modulate their microenvironment and facilitate angiogenesis and metastasis.
Figures


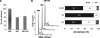

Similar articles
-
Non-coding RNAs shuttled via exosomes reshape the hypoxic tumor microenvironment.J Hematol Oncol. 2020 Jun 5;13(1):67. doi: 10.1186/s13045-020-00893-3. J Hematol Oncol. 2020. PMID: 32503591 Free PMC article. Review.
-
Role of hypoxia-induced exosomes in tumor biology.Mol Cancer. 2018 Aug 11;17(1):120. doi: 10.1186/s12943-018-0869-y. Mol Cancer. 2018. PMID: 30098600 Free PMC article. Review.
-
Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced β-Catenin Signaling in Endothelial Cells.Oncol Res. 2017 May 24;25(5):651-661. doi: 10.3727/096504016X14752792816791. Epub 2016 Oct 5. Oncol Res. 2017. PMID: 27712599 Free PMC article.
-
Exosomes in Tumor Angiogenesis.Methods Mol Biol. 2016;1464:25-34. doi: 10.1007/978-1-4939-3999-2_3. Methods Mol Biol. 2016. PMID: 27858353
-
Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules.Mol Carcinog. 2015 Jul;54(7):554-65. doi: 10.1002/mc.22124. Epub 2013 Dec 17. Mol Carcinog. 2015. PMID: 24347249 Free PMC article.
Cited by
-
Extracellular signals regulate the biogenesis of extracellular vesicles.Biol Res. 2022 Nov 26;55(1):35. doi: 10.1186/s40659-022-00405-2. Biol Res. 2022. PMID: 36435789 Free PMC article. Review.
-
Exosomes Derived from Hypoxic Colorectal Cancer Cells Transfer miR-410-3p to Regulate Tumor Progression.J Cancer. 2020 May 25;11(16):4724-4735. doi: 10.7150/jca.33232. eCollection 2020. J Cancer. 2020. PMID: 32626519 Free PMC article.
-
Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi.Int J Mol Sci. 2013 May 2;14(5):9581-603. doi: 10.3390/ijms14059581. Int J Mol Sci. 2013. PMID: 23644887 Free PMC article. Review.
-
Targeted inhibition of tumor-derived exosomes as a novel therapeutic option for cancer.Exp Mol Med. 2022 Sep;54(9):1379-1389. doi: 10.1038/s12276-022-00856-3. Epub 2022 Sep 18. Exp Mol Med. 2022. PMID: 36117219 Free PMC article. Review.
-
Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications.Int J Mol Sci. 2013 Mar 6;14(3):5338-66. doi: 10.3390/ijms14035338. Int J Mol Sci. 2013. PMID: 23466882 Free PMC article.
References
-
- Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 - PubMed
-
- Stacker S. A., Baldwin M. E., Achen M. G. (2002) The role of tumor lymphangiogenesis in metastatic spread. FASEB J 16, 922–934 - PubMed
-
- Sporn M. B. (1997) The war on cancer: a review. Ann. N.Y. Acad. Sci 833, 137–146 - PubMed
-
- Ferrara N., Hillan K. J., Novotny W. (2005) Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun 333, 328–335 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

