Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial
- PMID: 20124230
- PMCID: PMC3782858
- DOI: 10.7326/0003-4819-152-3-201002020-00005
Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial
Abstract
Background: Tobacco dependence is a chronic, relapsing condition that may require extended treatment.
Objective: To assess whether extended-duration transdermal nicotine therapy increases abstinence from tobacco more than standard-duration therapy in adult smokers.
Design: Parallel randomized, placebo-controlled trial from September 2004 to February 2008. Participants and all research personnel except the database manager were blinded to randomization. (ClinicalTrials.gov registration number: NCT00364156)
Setting: Academic center.
Participants: 568 adult smokers.
Intervention: In an unstratified small block-randomization scheme, participants were randomly assigned to standard therapy (Nicoderm CQ [GlaxoSmithKline, Research Triangle Park, North Carolina], 21 mg, for 8 weeks and placebo for 16 weeks) or extended therapy (Nicoderm CQ, 21 mg, for 24 weeks).
Measurements: The primary outcome was biochemically confirmed point-prevalence abstinence at weeks 24 and 52. Secondary outcomes were continuous and prolonged abstinence, lapse and recovery events, cost per additional quitter, and side effects and adherence.
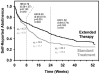
Results: At week 24, extended therapy produced higher rates of point-prevalence abstinence (31.6% vs. 20.3%; odds ratio, 1.81 [95% CI, 1.23 to 2.66]; P = 0.002), prolonged abstinence (41.5% vs. 26.9%; odds ratio, 1.97 [CI, 1.38 to 2.82]; P = 0.001), and continuous abstinence (19.2% vs. 12.6%; odds ratio, 1.64 [CI, 1.04 to 2.60]; P = 0.032) versus standard therapy. Extended therapy reduced the risk for lapse (hazard ratio, 0.77 [CI, 0.63 to 0.95]; P = 0.013) and increased the chances of recovery from lapses (hazard ratio, 1.47 [CI, 1.17 to 1.84]; P = 0.001). Time to relapse was slower with extended versus standard therapy (hazard ratio, 0.50 [CI, 0.35 to 0.73]; P < 0.001). At week 52, extended therapy produced higher quit rates for prolonged abstinence only (P = 0.027). No differences in side effects and adverse events between groups were found at the extended-treatment assessment.
Limitation: The generalizability of the findings may be limited because participants were smokers without medical comorbid conditions who were seeking treatment, and differences in adherence across treatment groups were detected.
Conclusion: Transdermal nicotine for 24 weeks increased biochemically confirmed point-prevalence abstinence and continuous abstinence at week 24, reduced the risk for smoking lapses, and increased the likelihood of recovery to abstinence after a lapse compared with 8 weeks of transdermal nicotine therapy.
Primary funding source: National Institutes of Health.
Conflict of interest statement
Figures
Comment in
-
Extended-duration transdermal nicotine therapy was more effective than standard-duration therapy for smoking cessation.Ann Intern Med. 2010 Apr 20;152(8):JC4-8. doi: 10.7326/0003-4819-152-8-201004200-02008. Ann Intern Med. 2010. PMID: 20404377 No abstract available.
-
Extending transdermal nicotine therapy from 8 to 24 weeks increases point-prevalence abstinence at 24 but not 52 weeks.Evid Based Med. 2010 Jun;15(3):87-8. doi: 10.1136/ebm1064. Evid Based Med. 2010. PMID: 20522690 No abstract available.
Summary for patients in
-
Summaries for patients: Extended nicotine treatment for long-term smokers.Ann Intern Med. 2010 Feb 2;152(3):I38. doi: 10.7326/0003-4819-152-3-201002020-00002. Ann Intern Med. 2010. PMID: 20124226 No abstract available.
References
-
- Jonk YC, Sherman SE, Fu SS, Hamlett-Berry KW, Geraci MC, Joseph AM. National trends in the provision of smoking cessation aids within the Veterans Health Administration. Am J Manag Care. 2005;11:77–85. - PubMed
-
- Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288:1260–4. - PubMed
-
- Tilson L, Bennett K, Barry M. Prescribing trends for nicotine replacement therapy in primary care. Ir Med J. 2004;97:270–3. - PubMed
-
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; May, 2008. Treating tobacco use and dependence: 2008 Update. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care 2000; 45:1196-9.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
