Endosymbiotic associations within protists
- PMID: 20124339
- PMCID: PMC2817226
- DOI: 10.1098/rstb.2009.0188
Endosymbiotic associations within protists
Abstract
The establishment of an endosymbiotic relationship typically seems to be driven through complementation of the host's limited metabolic capabilities by the biochemical versatility of the endosymbiont. The most significant examples of endosymbiosis are represented by the endosymbiotic acquisition of plastids and mitochondria, introducing photosynthesis and respiration to eukaryotes. However, there are numerous other endosymbioses that evolved more recently and repeatedly across the tree of life. Recent advances in genome sequencing technology have led to a better understanding of the physiological basis of many endosymbiotic associations. This review focuses on endosymbionts in protists (unicellular eukaryotes). Selected examples illustrate the incorporation of various new biochemical functions, such as photosynthesis, nitrogen fixation and recycling, and methanogenesis, into protist hosts by prokaryotic endosymbionts. Furthermore, photosynthetic eukaryotic endosymbionts display a great diversity of modes of integration into different protist hosts. In conclusion, endosymbiosis seems to represent a general evolutionary strategy of protists to acquire novel biochemical functions and is thus an important source of genetic innovation.
Figures

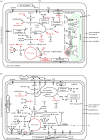
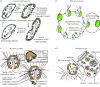
References
-
- Akman L., Yamashita A., Watanabe H., Oshima K., Shiba T., Hattori M., Aksoy S.2002Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 32, 402–407 (doi:10.1038/ng986) - DOI - PubMed
-
- Andersson J. O.2005Lateral gene transfer in eukaryotes. Cell. Mol. Life Sci. 62, 1182–1197 (doi:10.1007/s00018-005-4539-z) - DOI - PMC - PubMed
-
- Archibald J. M.2009The puzzle of plastid evolution. Curr. Biol. 19, R81–R88 (doi:10.1016/j.cub.2008.11.067) - DOI - PubMed
-
- Baumann P.2005Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59, 155–189 (doi:10.1146/annurev.micro.59.030804.121041) - DOI - PubMed
-
- Bhattacharya D., Helmchen T., Melkonian M.1995Molecular evolutionary analyses of nuclear-encoded small-subunit ribosomal RNA identify an independent rhizopod lineage containing the Euglyphina and the Chlorarachniophyta. J. Eukaryot. Microbiol. 42, 65–69 (doi:10.1111/j.1550-7408.1995.tb01541.x) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
