A docking model based on mass spectrometric and biochemical data describes phage packaging motor incorporation
- PMID: 20124351
- PMCID: PMC2938058
- DOI: 10.1074/mcp.M900625-MCP200
A docking model based on mass spectrometric and biochemical data describes phage packaging motor incorporation
Abstract
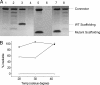
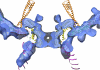
The molecular mechanism of scaffolding protein-mediated incorporation of one and only one DNA packaging motor/connector dodecamer at a unique vertex during lambdoid phage assembly has remained elusive because of the lack of structural information on how the connector and scaffolding proteins interact. We assembled and characterized a phi29 connector-scaffolding complex, which can be incorporated into procapsids during in vitro assembly. Native mass spectrometry revealed that the connector binds at most 12 scaffolding molecules, likely organized as six dimers. A data-driven docking model, using input from chemical cross-linking and mutagenesis data, suggested an interaction between the scaffolding protein and the exterior of the wide domain of the connector dodecamer. The connector binding region of the scaffolding protein lies upstream of the capsid binding region located at the C terminus. This arrangement allows the C terminus of scaffolding protein within the complex to both recruit capsid subunits and mediate the incorporation of the single connector vertex.
Figures

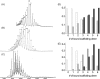



Similar articles
-
In vitro incorporation of the phage Phi29 connector complex.Virology. 2009 Nov 10;394(1):149-53. doi: 10.1016/j.virol.2009.08.016. Epub 2009 Sep 9. Virology. 2009. PMID: 19744688 Free PMC article.
-
Binding of pRNA to the N-terminal 14 amino acids of connector protein of bacteriophage phi29.Nucleic Acids Res. 2005 May 10;33(8):2640-9. doi: 10.1093/nar/gki554. Print 2005. Nucleic Acids Res. 2005. PMID: 15886394 Free PMC article.
-
Crystal structure of 3WJ core revealing divalent ion-promoted thermostability and assembly of the Phi29 hexameric motor pRNA.RNA. 2013 Sep;19(9):1226-37. doi: 10.1261/rna.037077.112. Epub 2013 Jul 24. RNA. 2013. PMID: 23884902 Free PMC article.
-
Construction of bacteriophage phi29 DNA packaging motor and its applications in nanotechnology and therapy.Ann Biomed Eng. 2009 Oct;37(10):2064-81. doi: 10.1007/s10439-009-9723-0. Epub 2009 Jun 4. Ann Biomed Eng. 2009. PMID: 19495981 Free PMC article. Review.
-
Structure and function of phi29 hexameric RNA that drives the viral DNA packaging motor: review.Prog Nucleic Acid Res Mol Biol. 2002;72:415-72. doi: 10.1016/s0079-6603(02)72076-x. Prog Nucleic Acid Res Mol Biol. 2002. PMID: 12206459 Review.
Cited by
-
Mind the gap: how some viruses infect their hosts.Viruses. 2010 Nov;2(11):2536-40. doi: 10.3390/v2112536. Epub 2010 Nov 12. Viruses. 2010. PMID: 21994629 Free PMC article.
-
Structure of the Portal Complex from Staphylococcus aureus Pathogenicity Island 1 Transducing Particles In Situ and In Isolation.J Mol Biol. 2024 Feb 15;436(4):168415. doi: 10.1016/j.jmb.2023.168415. Epub 2023 Dec 21. J Mol Biol. 2024. PMID: 38135177 Free PMC article.
-
Structure and host specificity of Staphylococcus epidermidis bacteriophage Andhra.Sci Adv. 2022 Dec 2;8(48):eade0459. doi: 10.1126/sciadv.ade0459. Epub 2022 Nov 30. Sci Adv. 2022. PMID: 36449623 Free PMC article.
-
Fluorescence, Circular Dichroism and Mass Spectrometry as Tools to Study Virus Structure.Subcell Biochem. 2024;105:207-245. doi: 10.1007/978-3-031-65187-8_6. Subcell Biochem. 2024. PMID: 39738948 Review.
-
Cryo-EM Data Are Superior to Contact and Interface Information in Integrative Modeling.Biophys J. 2016 Feb 23;110(4):785-97. doi: 10.1016/j.bpj.2015.12.038. Epub 2016 Feb 1. Biophys J. 2016. PMID: 26846888 Free PMC article.
References
-
- Smith D. E., Tans S. J., Smith S. B., Grimes S., Anderson D. L., Bustamante C. (2001) The bacteriophage straight phi29 portal motor can package DNA against a large internal force. Nature 413, 748–752 - PubMed
-
- Bazinet C., King J. (1985) The DNA translocating vertex of dsDNA bacteriophage. Annu. Rev. Microbiol. 39, 109–129 - PubMed
-
- Moore S. D., Prevelige P. E., Jr (2002) Bacteriophage p22 portal vertex formation in vivo. J. Mol. Biol. 315, 975–994 - PubMed
-
- Casjens S., King J. (1974) P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J. Supramol. Struct. 2, 202–224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

