Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis
- PMID: 20124436
- PMCID: PMC2874481
- DOI: 10.1096/fj.09-144659
Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis
Abstract
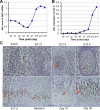
Granulin epithelin precursor (GEP) has been implicated in development, tissue regeneration, tumorigenesis, and inflammation. Herein we report that GEP stimulates chondrocyte differentiation from mesenchymal stem cells in vitro and endochondral ossification ex vivo, and GEP-knockdown mice display skeleton defects. Similar to bone morphogenic protein (BMP) 2, application of the recombinant GEP accelerates rabbit cartilage repair in vivo. GEP is a key downstream molecule of BMP2, and it is required for BMP2-mediated chondrocyte differentiation. We also show that GEP activates chondrocyte differentiation through Erk1/2 signaling and that JunB transcription factor is one of key downstream molecules of GEP in chondrocyte differentiation. Collectively, these findings reveal a novel critical role of GEP growth factor in chondrocyte differentiation and the molecular events both in vivo and in vitro.
Figures
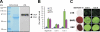



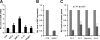

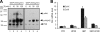
Similar articles
-
XBP1S, a BMP2-inducible transcription factor, accelerates endochondral bone growth by activating GEP growth factor.J Cell Mol Med. 2014 Jun;18(6):1157-71. doi: 10.1111/jcmm.12261. Epub 2014 Mar 18. J Cell Mol Med. 2014. PMID: 24636354 Free PMC article.
-
IRE1a constitutes a negative feedback loop with BMP2 and acts as a novel mediator in modulating osteogenic differentiation.Cell Death Dis. 2014 May 22;5(5):e1239. doi: 10.1038/cddis.2014.194. Cell Death Dis. 2014. PMID: 24853417 Free PMC article.
-
ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor.Mol Cell Biol. 2009 Aug;29(15):4201-19. doi: 10.1128/MCB.00056-09. Epub 2009 Jun 1. Mol Cell Biol. 2009. PMID: 19487464 Free PMC article.
-
Bmpr1a Signaling in Cartilage Development and Endochondral Bone Formation.Vitam Horm. 2015;99:273-91. doi: 10.1016/bs.vh.2015.06.001. Epub 2015 Jul 15. Vitam Horm. 2015. PMID: 26279380 Review.
-
Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling.Birth Defects Res C Embryo Today. 2008 Jun;84(2):131-54. doi: 10.1002/bdrc.20126. Birth Defects Res C Embryo Today. 2008. PMID: 18546337 Review.
Cited by
-
The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling.Biomaterials. 2013 Sep;34(27):6412-21. doi: 10.1016/j.biomaterials.2013.05.030. Epub 2013 Jun 5. Biomaterials. 2013. PMID: 23746860 Free PMC article.
-
Proepithelin stimulates growth plate chondrogenesis via nuclear factor-kappaB-p65-dependent mechanisms.J Biol Chem. 2011 Jul 8;286(27):24057-67. doi: 10.1074/jbc.M110.201368. Epub 2011 May 12. J Biol Chem. 2011. Retraction in: J Biol Chem. 2020 Jun 26;295(26):8874. doi: 10.1074/jbc.W120.014401. PMID: 21566130 Free PMC article. Retracted.
-
Transmission of ER stress response by ATF6 promotes endochondral bone growth.J Orthop Surg Res. 2015 Sep 15;10:141. doi: 10.1186/s13018-015-0284-7. J Orthop Surg Res. 2015. PMID: 26374329 Free PMC article.
-
Lysosomal Dysfunction and Other Pathomechanisms in FTLD: Evidence from Progranulin Genetics and Biology.Adv Exp Med Biol. 2021;1281:219-242. doi: 10.1007/978-3-030-51140-1_14. Adv Exp Med Biol. 2021. PMID: 33433878 Free PMC article.
-
Cellular effects of progranulin in health and disease.J Mol Neurosci. 2011 Nov;45(3):549-60. doi: 10.1007/s12031-011-9553-z. Epub 2011 May 25. J Mol Neurosci. 2011. PMID: 21611805 Review.
References
-
- Colnot C. Cellular and molecular interactions regulating skeletogenesis. J Cell Biochem. 2005;95:688–697. - PubMed
-
- Franz-Odendaal T A, Vickaryous M K. Skeletal elements in the vertebrate eye and adnexa: morphological and developmental perspectives. Dev Dyn. 2006;235:1244–1255. - PubMed
-
- Goldring M B, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. - PubMed
-
- Ornitz D M, Marie P J. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. - PubMed
-
- Tuan R S. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. J Bone Joint Surg Am. 2003;85A:137–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

