Reprogramming murine telomerase rapidly inhibits the growth of mouse cancer cells in vitro and in vivo
- PMID: 20124445
- PMCID: PMC2820598
- DOI: 10.1158/1535-7163.MCT-09-0682
Reprogramming murine telomerase rapidly inhibits the growth of mouse cancer cells in vitro and in vivo
Abstract
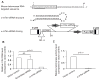
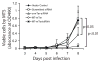
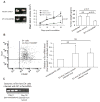
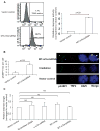
Telomerase plays a critical role in cancer, prompting the pursuit of various telomerase-based therapeutic strategies. One such strategy, telomerase interference, exploits the high telomerase activity in cancer cells and reprograms telomerase to encode "toxic" telomeres. To date, telomerase interference has been tested in human cancer cells xenografted into mice, an approach that does not recapitulate spontaneous malignancy and offers few insights about host toxicities, because human telomerase is targeted in a mouse host. To address these limitations, we designed and validated two new gene constructs specifically targeting mouse telomerase: mutant template mouse telomerase RNA (MT-mTer) and small interfering RNA against wild-type mouse telomerase RNA (α-mTer-siRNA). Using lentiviral delivery in mouse prostate cancer cells, we achieved α-mTer-siRNA-mediated knockdown of wild-type mTer (80% depletion) and concurrent overexpression of MT-mTer (50-fold). We showed that the two constructs effectively synergize to reprogram murine telomerase to add mutant instead of wild-type telomeric repeats, resulting in rapid telomeric uncapping (5-fold increase in DNA damage foci). This, in turn, led to rapid and significant apoptosis (>90% of cells) and growth inhibition in vitro (90% reduction in viable cell mass) and in vivo (75% reduction in tumor allograft wet weight). In summary, we have shown that mouse cancer cells are vulnerable to direct telomerase interference using novel murine telomerase-targeting constructs; this approach can now be used to study the true therapeutic potential of telomerase interference in mouse spontaneous cancer models.
Figures

Similar articles
-
Assembly of mutant-template telomerase RNA into catalytically active telomerase ribonucleoprotein that can act on telomeres is required for apoptosis and cell cycle arrest in human cancer cells.Cancer Res. 2006 Jun 1;66(11):5763-71. doi: 10.1158/0008-5472.CAN-05-3782. Cancer Res. 2006. PMID: 16740715
-
Antitumor activity of systemically delivered ribozymes targeting murine telomerase RNA.Clin Cancer Res. 2004 Aug 1;10(15):4983-90. doi: 10.1158/1078-0432.CCR-04-0134. Clin Cancer Res. 2004. PMID: 15297398
-
ATM mediates cytotoxicity of a mutant telomerase RNA in human cancer cells.Cancer Res. 2008 Jul 1;68(13):5309-17. doi: 10.1158/0008-5472.CAN-08-0504. Cancer Res. 2008. PMID: 18593932 Free PMC article.
-
[siRNA targeting telomerase--effective tool in anti-cancer therapy?].Postepy Biochem. 2008;54(4):384-92. Postepy Biochem. 2008. PMID: 19248585 Review. Polish.
-
Overcoming the immortality of tumour cells by telomere and telomerase based cancer therapeutics--current status and future prospects.Eur J Cancer. 2005 May;41(7):971-9. doi: 10.1016/j.ejca.2004.11.024. Eur J Cancer. 2005. PMID: 15862745 Review.
Cited by
-
Telomere and Telomerase Therapeutics in Cancer.Genes (Basel). 2016 May 26;7(6):22. doi: 10.3390/genes7060022. Genes (Basel). 2016. PMID: 27240403 Free PMC article. Review.
-
Cancer cells cyclically lose and regain drug-resistant highly tumorigenic features characteristic of a cancer stem-like phenotype.Mol Cancer Ther. 2011 Jun;10(6):938-48. doi: 10.1158/1535-7163.MCT-10-1120. Epub 2011 Apr 25. Mol Cancer Ther. 2011. PMID: 21518726 Free PMC article.
-
A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter.Cancer Res. 2010 Aug 15;70(16):6420-6. doi: 10.1158/0008-5472.CAN-10-0686. Epub 2010 Jul 27. Cancer Res. 2010. PMID: 20663903 Free PMC article.
-
Prostate tumor cells with cancer progenitor properties have high telomerase activity and are rapidly killed by telomerase interference.Prostate. 2011 Sep 15;71(13):1390-400. doi: 10.1002/pros.21355. Epub 2011 Feb 14. Prostate. 2011. PMID: 21321978 Free PMC article.
-
Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG 0421: a phase III metastatic castration resistant prostate cancer trial.Int J Cancer. 2015 Apr 15;136(8):1856-62. doi: 10.1002/ijc.29212. Epub 2014 Oct 8. Int J Cancer. 2015. PMID: 25219358 Free PMC article. Clinical Trial.
References
-
- T De Lange VL. EH Blackburn Telomere. 2. New York: Cold Spring Harbor Laboratory Press; 2005.
-
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. - PubMed
-
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. - PubMed
-
- Meeker AK. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol Oncol. 2006;24:122–130. - PubMed
-
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:87–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

