Serologic markers of effective tumor immunity against chronic lymphocytic leukemia include nonmutated B-cell antigens
- PMID: 20124481
- PMCID: PMC2852266
- DOI: 10.1158/0008-5472.CAN-09-3143
Serologic markers of effective tumor immunity against chronic lymphocytic leukemia include nonmutated B-cell antigens
Abstract
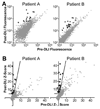
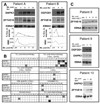
Patients with chronic lymphocytic leukemia (CLL) who relapse after allogeneic transplant may achieve durable remission following donor lymphocyte infusion (DLI), showing the potency of donor-derived immunity in eradicating tumors. We sought to elucidate the antigenic basis of the effective graft-versus-leukemia (GvL) responses associated with DLI for the treatment of CLL by analyzing the specificity of plasma antibody responses developing in two DLI-treated patients who achieved long-term remission without graft-versus-host disease. By probing high-density protein microarrays with patient plasma, we discovered 35 predominantly intracellular antigens that elicited high-titer antibody reactivity greater in post-DLI than in pre-DLI plasma. Three antigens-C6orf130, MDS032, and ZFYVE19-were identified by both patients. Along with additional candidate antigens DAPK3, SERBP1, and OGFOD1, these proteins showed higher transcript and protein expression in B cells and CLL cells compared with normal peripheral blood mononuclear cells. DAPK3 and the shared antigens do not represent minor histocompatibility antigens, as their sequences are identical in both donor and tumor. Although ZFYVE19, DAPK3, and OGFOD1 elicited minimal antibody reactivity in 12 normal subjects and 12 chemotherapy-treated CLL patients, 5 of 12 CLL patients with clinical GvL responses were serologically reactive to these antigens. Moreover, antibody reactivity against these antigens was temporally correlated with clinical disease regression. These B-cell antigens represent promising biomarkers of effective anti-CLL immunity.
Figures
 other protein.
other protein.

References
-
- Byrd JC, Lin TS, Grever MR. Treatment of relapsed chronic lymphocytic leukemia: old and new therapies. Semin Oncol. 2006;33:210–219. - PubMed
-
- Esteve J, Villamor N, Colomer D, et al. Stem cell transplantation for chronic lymphocytic leukemia: different outcome after autologous and allogeneic transplantation and correlation with minimal residual disease status. Leukemia. 2001;15:445–451. - PubMed
-
- Dreger P, Corradini P, Kimby E, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12–17. - PubMed
-
- Pavletic SZ, Khouri IF, Haagenson M, et al. Unrelated donor marrow transplantation for B-cell chronic lymphocytic leukemia after using myeloablative conditioning: results from the Center for International Blood and Marrow Transplant research. J Clin Oncol. 2005;23:5788–5794. - PubMed
-
- Rondon G, Giralt S, Huh Y, et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant. 1996;18:669–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

