Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells
- PMID: 20124487
- PMCID: PMC2822899
- DOI: 10.1158/0008-5472.CAN-09-2612
Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells
Abstract
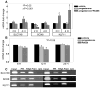
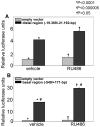
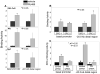
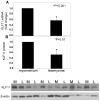
Uterine leiomyoma is the most common tumor of the female genital tract and the leading cause of hysterectomy. Although progesterone stimulates the proliferation of uterine leiomyoma cells, the mechanism of progesterone action is not well understood. We used chromatin immunoprecipitation (ChIP)-cloning approach to identify progesterone receptor (PR) target genes in primary uterine leiomyoma smooth muscle cells. We identified 18 novel PR-binding sites, one of which was located 20.5 kb upstream of the transcriptional start site of the Krüppel-like transcription factor 11 (KLF11) gene. KLF11 mRNA levels were minimally downregulated by progesterone but robustly upregulated by the progesterone antagonist RU486. Luciferase reporter assays showed significant baseline and RU486-inducible promoter activity in the KLF11 basal promoter or distal PR-binding region, both of which contained multiple Sp1-binding sequences but lacked classic progesterone response elements. RU486 stimulated recruitment of Sp1, RNA polymerase II, PR, and the coactivators SRC-1 and SRC-2 to the distal region and basal promoter. siRNA knockdown of PR increased KLF11 expression, whereas knockdown of KLF11 increased leiomyoma cell proliferation and abolished the antiproliferative effect of RU486. In vivo, KLF11 expression was significantly lower in leiomyoma tissues compared with adjacent myometrial tissues. Taken together, using a ChIP-cloning approach, we uncovered KLF11 as an integrator of PR signaling and proliferation in uterine leiomyoma cells.
Figures


References
-
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–38. - PubMed
-
- Kovacs KA, Lengyel F, Kornyei JL, et al. Differential expression of Akt/protein kinase B, Bcl-2 and Bax proteins in human leiomyoma and myometrium. J Steroid Biochem Mol Biol. 2003;87:233–40. - PubMed
-
- Brandon DD, Bethea CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. - PubMed
-
- Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637–41. - PubMed
-
- Tiltman AJ. The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Pathol. 1985;4:89–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

