Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance
- PMID: 20124509
- PMCID: PMC2879104
- DOI: 10.1182/blood-2009-11-255620
Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance
Abstract
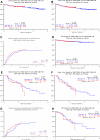
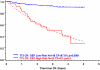
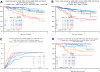
The Total Therapy 3 trial 2003-33 enrolled 303 newly diagnosed multiple myeloma patients and was noted to provide superior clinical outcomes compared with predecessor trial Total Therapy 2, especially in gene expression profiling (GEP)-defined low-risk disease. We report here on the results of successor trial 2006-66 with 177 patients, using bortezomib, lenalidomide, and dexamethasone maintenance for 3 years versus bortezomib, thalidomide, and dexamethasone in year 1 and thalidomide/dexamethasone in years 2 and 3 in the 2003-33 protocol. Overall survival (OS) and event-free survival (EFS) plots were super-imposable for the 2 trials, as were onset of complete response and complete response duration (CRD), regardless of GEP risk. GEP-defined high-risk designation, pertinent to 17% of patients, imparted inferior OS, EFS, and CRD in both protocols and, on multivariate analysis, was the sole adverse feature affecting OS, EFS, and CRD. Mathematical modeling of CRD in low-risk myeloma predicted a 55% cure fraction (P < .001). Despite more rapid onset and higher rate of CR than in other molecular subgroups, CRD was inferior in CCND1 without CD20 myeloma, resembling outcomes in MAF/MAFB and proliferation entities. The robustness of the GEP risk model should be exploited in clinical trials aimed at improving the notoriously poor outcome in high-risk disease.
Figures

Comment in
-
Refining "total therapy" for myeloma.Blood. 2010 May 27;115(21):4152-3. doi: 10.1182/blood-2010-02-271338. Blood. 2010. PMID: 20508167 No abstract available.
References
-
- Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138(2):176–185. - PubMed
-
- Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354(10):1021–1030. - PubMed
-
- Shaughnessy JD, Jr, Qu P, Edmondson R, et al. Changes in the expression of proteasome genes in tumor cells following short-term proteasome inhibitor therapy predicts survival in multiple myeloma treated with bortezomib-containing multi-agent chemotherapy. Blood (ASH Annual Meeting Abstracts) 2008;112 Abstract 733.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

