c-Abl mediates endothelial apoptosis induced by inhibition of integrins alphavbeta3 and alphavbeta5 and by disruption of actin
- PMID: 20124512
- PMCID: PMC2852370
- DOI: 10.1182/blood-2009-05-223776
c-Abl mediates endothelial apoptosis induced by inhibition of integrins alphavbeta3 and alphavbeta5 and by disruption of actin
Abstract
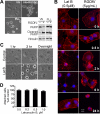
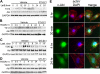
Inhibition of integrins alphavbeta3 and alphavbeta5 in human brain microvascular endothelial cells (HBMECs) by the function-blocking peptide RGDfV induces loss of spreading on vitronectin, cell detachment, and apoptosis. We demonstrate that cell detachment is not required for apoptosis because plating on bovine serum albumin-blocked poly-L-lysine (allows attachment, but not integrin ligation and cell spreading) also induced apoptosis. Latrunculin B (LatB), which inhibits F-actin polymerization, induced transient loss of HBMEC spreading on vitronectin, but not their detachment, and induced apoptosis despite recovery of cell spreading. However, LatB did not cause apoptosis in 5 tumor cell lines. In HBMECs, both LatB and RGDfV induced transient Y412 and Y245 phosphorylation of endogenous c-Abl, a nonreceptor tyrosine kinase that reciprocally regulates F-actin. LatB also induced nuclear translocation of c-Abl in HBMECs. STI-571 (imatinib), a targeted therapy for BCR-ABL1(+) leukemias and inhibitor of c-Abl, platelet-derived growth factor receptor, and c-Kit, decreased endothelial apoptosis. LatB-induced HBMEC apoptosis, and its inhibition by STI-571 also occurred in a 3-dimensional collagen model, supporting physiologic relevance. Last, siRNA to c-Abl (but not nonspecific siRNA) also inhibited RGDfV- and LatB-induced apoptosis. Thus, endogenous c-Abl mediates endothelial apoptosis induced by inhibition of integrins alphavbeta3/alphavbeta5 or by LatB-induced disruption of F-actin.
Figures




Similar articles
-
c-Abl is an upstream regulator of acid sphingomyelinase in apoptosis induced by inhibition of integrins αvβ3 and αvβ5.PLoS One. 2012;7(8):e42291. doi: 10.1371/journal.pone.0042291. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879933 Free PMC article.
-
Endothelial apoptosis induced by inhibition of integrins alphavbeta3 and alphavbeta5 involves ceramide metabolic pathways.Blood. 2005 Jun 1;105(11):4353-61. doi: 10.1182/blood-2004-08-3098. Epub 2005 Feb 10. Blood. 2005. PMID: 15705795 Free PMC article.
-
A selective cyclic integrin antagonist blocks the integrin receptors alphavbeta3 and alphavbeta5 and inhibits retinal pigment epithelium cell attachment, migration and invasion.BMC Ophthalmol. 2005 Jun 29;5:16. doi: 10.1186/1471-2415-5-16. BMC Ophthalmol. 2005. PMID: 15987521 Free PMC article.
-
c-Abl Kinase Is a Regulator of αvβ3 Integrin Mediated Melanoma A375 Cell Migration.PLoS One. 2013 Jun 21;8(6):e66108. doi: 10.1371/journal.pone.0066108. Print 2013. PLoS One. 2013. PMID: 23805201 Free PMC article.
-
Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases.J Cell Sci. 2003 Jul 1;116(Pt 13):2613-26. doi: 10.1242/jcs.00622. J Cell Sci. 2003. PMID: 12775773 Review.
Cited by
-
c-Abl is an upstream regulator of acid sphingomyelinase in apoptosis induced by inhibition of integrins αvβ3 and αvβ5.PLoS One. 2012;7(8):e42291. doi: 10.1371/journal.pone.0042291. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879933 Free PMC article.
-
Lyn facilitates glioblastoma cell survival under conditions of nutrient deprivation by promoting autophagy.PLoS One. 2013 Aug 2;8(8):e70804. doi: 10.1371/journal.pone.0070804. Print 2013. PLoS One. 2013. PMID: 23936469 Free PMC article.
-
GRK2 promotes growth of medulloblastoma cells and protects them from chemotherapy-induced apoptosis.Sci Rep. 2019 Sep 25;9(1):13902. doi: 10.1038/s41598-019-50157-5. Sci Rep. 2019. PMID: 31554835 Free PMC article.
-
Abl Tyrosine Kinase Regulates Hepatitis C Virus Entry.Front Microbiol. 2017 Jun 19;8:1129. doi: 10.3389/fmicb.2017.01129. eCollection 2017. Front Microbiol. 2017. PMID: 28674529 Free PMC article.
-
Understanding mechanism of in vitro maturation, fertilization and culture of sheep embryoes through in silico analysis.Bioinformation. 2012;8(21):1030-4. doi: 10.6026/97320630081030. Epub 2012 Oct 31. Bioinformation. 2012. PMID: 23275702 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

