Signaling actions of electrophiles: anti-inflammatory therapeutic candidates
- PMID: 20124562
- PMCID: PMC3139380
- DOI: 10.1124/mi.10.1.7
Signaling actions of electrophiles: anti-inflammatory therapeutic candidates
Abstract
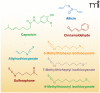
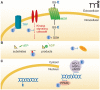
Over the past several years, research on biologically relevant electrophiles has been replete with new insights, expanding our understanding of the roles electrophiles play in vivo. Importantly, many electrophiles can form reversible covalent adducts with both proteins and small-molecule thiols in cells. This post-translational protein modification has important ramifications, including changes in protein enzymatic activity, the transduction of signals within and between cells, and alterations in gene expression. Electrophiles modulate a variety of cellular signaling processes that are involved in several major diseases with inflammatory components. The electrophilic fatty-acid derivatives discussed in this work are naturally occurring products of redox reactions and enzymatic activity. Furthermore, several of these electrophilic species and their derivatives represent potential therapeutic candidates.
Figures





References
-
- LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: Relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. This article explains the term “soft electrophile” for α,β unsaturated carbonyl, based on calculated quantum mechanical parameters, and explores the involvement of environmental electrophile exposure in neurodegenerative diseases. - PMC - PubMed
-
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. - PubMed
-
- Moos PJ, Edes K, Cassidy P, Massuda E, Fitzpatrick FA. Electrophilic prostaglandins and lipid aldehydes repress redox-sensitive transcription factors p53 and hypoxia-inducible factor by impairing the selenoprotein thioredoxin reductase. J Biol Chem. 2003;278:745–750. This is a definitive study on how α,β unsaturated carbonyls impair thioredoxin reductase activity by adducting a crucial seleno-cysteine residue and thus contributing to p53 dysfunction. - PubMed
-
- Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. - PubMed
-
- Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103:15404–15409. This study linked acrolein adduction to DNA within the p53 gene to cigarette smoke-related lung cancer. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
