Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms
- PMID: 20124983
- PMCID: PMC2888104
- DOI: 10.1097/ALN.0b013e3181cded1f
Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms
Abstract
Background: Endothelial nitric oxide synthase activity is regulated by (6R-)5,6,7,8-tetrahydrobiopterin (BH4) and heat shock protein 90. The authors tested the hypothesis that hyperglycemia abolishes anesthetic preconditioning (APC) through BH4- and heat shock protein 90-dependent pathways.
Methods: Myocardial infarct size was measured in rabbits in the absence or presence of APC (30 min of isoflurane), with or without hyperglycemia, and in the presence or absence of the BH4 precursor sepiapterin. Isoflurane-dependent nitric oxide production was measured (ozone chemiluminescence) in human coronary artery endothelial cells cultured in normal (5.5 mm) or high (20 mm) glucose conditions, with or without sepiapterin (10 or 100 microm).
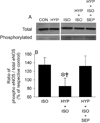
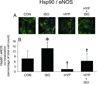
Results: APC decreased myocardial infarct size compared with control experiments (26 +/- 6% vs. 46 +/- 3%, respectively; P < 0.05), and this action was blocked by hyperglycemia (43 +/- 4%). Sepiapterin alone had no effect on infarct size (46 +/- 3%) but restored APC during hyperglycemia (21 +/- 3%). The beneficial actions of sepiapterin to restore APC were blocked by the nitric oxide synthase inhibitor N (G)-nitro-L-arginine methyl ester (47 +/- 2%) and the BH4 synthesis inhibitor N-acetylserotonin (46 +/- 3%). Isoflurane increased nitric oxide production to 177 +/- 13% of baseline, and this action was attenuated by high glucose concentrations (125 +/- 6%). Isoflurane increased, whereas high glucose attenuated intracellular BH4/7,8-dihydrobiopterin (BH2) (high performance liquid chromatography), heat shock protein 90-endothelial nitric oxide synthase colocalization (confocal microscopy) and endothelial nitric oxide synthase activation (immunoblotting). Sepiapterin increased BH4/BH2 and dose-dependently restored nitric oxide production during hyperglycemic conditions (149 +/- 12% and 175 +/- 9%; 10 and 100 microm, respectively).
Conclusion: The results indicate that tetrahydrobiopterin and heat shock protein 90-regulated endothelial nitric oxide synthase activity play a central role in cardioprotection that is favorably modulated by volatile anesthetics and dysregulated by hyperglycemia. Enhancing the production of BH4 may represent a potential therapeutic strategy.
Figures





References
-
- Amour J, Brzezinska AK, Weihrauch D, Billstrom AR, Zielonka J, Krolikowski JG, Bienengraeber MW, Warltier DC, Pratt PF, Jr, Kersten JR. Role of heat shock protein 90 and endothelial nitric oxide synthase during early anesthetic and ischemic preconditioning. Anesthesiology. 2009;110:317–325. - PMC - PubMed
-
- Chiari PC, Bienengraeber MW, Weihrauch D, Krolikowski JG, Kersten JR, Warltier DC, Pagel PS. Role of endothelial nitric oxide synthase as a trigger and mediator of isoflurane-induced delayed preconditioning in rabbit myocardium. Anesthesiology. 2005;103:74–83. - PubMed
-
- Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. - PubMed
-
- McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain "calcium-independent" eNOS activation by phosphorylation. J Biol Chem. 2000;275:6123–6128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

