The binary switch between life and death of endoplasmic reticulum-stressed beta cells
- PMID: 20125004
- PMCID: PMC2898716
- DOI: 10.1097/MED.0b013e3283372843
The binary switch between life and death of endoplasmic reticulum-stressed beta cells
Abstract
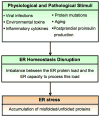
Purpose of review: beta-Cell death is an important pathogenic component of both type 1 and type 2 diabetes. However, the specific molecular pathways and interactions involved in this process are not completely understood. Increasing evidence indicates that a type of cell stress called endoplasmic reticulum stress (ER stress) plays an important role in beta-cell death. In the present article, we discuss a potential paradigm of ER stress-mediated beta-cell death.
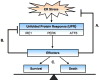
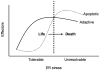
Recent findings: Upon ER stress conditions, a signaling network termed the unfolded protein response (UPR) is activated. The UPR regulates adaptive effectors to attenuate ER stress and restore ER homeostasis promoting cell survival. Paradoxically the UPR also regulates apoptotic effectors. When adaptive effectors fail to attenuate ER stress, these apoptotic effectors take into effect leading to cell death. The nature of this switch between life and death is currently under study.
Summary: Depending on the nature of the stress condition, the UPR either protects beta cells or promotes their death. The mechanisms of this switch are not well understood but involve the balance between adaptive and apoptotic factors regulated by the UPR. In the present article, we review examples of this UPR balancing act between life and death and the potential mechanisms involved.
Figures
References
-
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. - PubMed
-
- Rhodes CJ. Processing of the insulin molecule. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 27–50.
-
- Rhodes CJ, Shoelson S, Halban PA. Insulin Biosynthesis, Processing, and Chemistry. In: Kahn CR, Weir GC, King GL, et al., editors. Joslin’s Diabetes Mellitus. Boston: Joslin Diabetes Center; 2005. pp. 65–82.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

