Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries
- PMID: 20125181
- PMCID: PMC2949209
- DOI: 10.1038/jcbfm.2010.11
Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries
Abstract
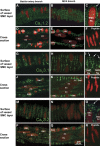
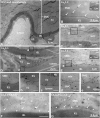
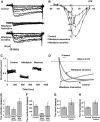
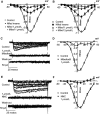
Although dihydropyridines are widely used for the treatment of vasospasm, their effectiveness is questionable, suggesting that other voltage-dependent calcium channels (VDCCs) contribute to control of cerebrovascular tone. This study therefore investigated the role of dihydropyridine-insensitive VDCCs in cerebrovascular function. Using quantitative PCR and immunohistochemistry, we found mRNA and protein for L-type (Ca(V)1.2) and T-type (Ca(V)3.1 and Ca(V)3.2) channels in adult rat basilar and middle cerebral arteries and their branches. Immunoelectron microscopy revealed both L- and T-type channels in smooth muscle cell (SMC) membranes. Using patch clamp electrophysiology, we found that a high-voltage-activated calcium current, showing T-type channel kinetics and insensitivity to nifedipine and nimodipine, comprised approximately 20% of current in SMCs of the main arteries and approximately 45% of current in SMCs from branches. Both components were abolished by the T-type antagonists mibefradil, NNC 55-0396, and efonidipine. Although nifedipine completely blocked vasoconstriction in pressurized basilar arteries, a nifedipine-insensitive constriction was found in branches and this increased in magnitude as vessel size decreased. We conclude that a heterogeneous population of VDCCs contributes to cerebrovascular function, with dihydropyridine-insensitive channels having a larger role in smaller vessels. Sensitivity of these currents to nonselective T-type channel antagonists suggests that these drugs may provide a more effective treatment for therapy-refractory cerebrovascular constriction.
Figures



Similar articles
-
Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development.Am J Physiol Heart Circ Physiol. 2013 Jan 1;304(1):H58-71. doi: 10.1152/ajpheart.00476.2012. Epub 2012 Oct 26. Am J Physiol Heart Circ Physiol. 2013. PMID: 23103495 Free PMC article.
-
Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage.Circ Res. 2005 Mar 4;96(4):419-26. doi: 10.1161/01.RES.0000157670.49936.da. Epub 2005 Feb 3. Circ Res. 2005. PMID: 15692089
-
Non-L-type voltage-dependent calcium channels control vascular tone of the rat basilar artery.Clin Exp Pharmacol Physiol. 2009 Jan;36(1):55-66. doi: 10.1111/j.1440-1681.2008.05035.x. Epub 2008 Aug 26. Clin Exp Pharmacol Physiol. 2009. PMID: 18759855
-
Vascular effects of calcium channel antagonists: new evidence.Drugs. 2005;65 Suppl 2:1-10. doi: 10.2165/00003495-200565002-00002. Drugs. 2005. PMID: 16398057 Review.
-
[Molecular effects of new calcium antagonists: is the principle of parcimony out of place?].Ann Cardiol Angeiol (Paris). 2008 Jun;57(3):166-73. doi: 10.1016/j.ancard.2008.02.019. Epub 2008 Jun 4. Ann Cardiol Angeiol (Paris). 2008. PMID: 18565491 Review. French.
Cited by
-
The Calcium Signaling Mechanisms in Arterial Smooth Muscle and Endothelial Cells.Compr Physiol. 2021 Apr 1;11(2):1831-1869. doi: 10.1002/cphy.c200030. Compr Physiol. 2021. PMID: 33792900 Free PMC article.
-
Reactive Oxygen Species Mediate the Suppression of Arterial Smooth Muscle T-type Ca2+ Channels by Angiotensin II.Sci Rep. 2018 Feb 22;8(1):3445. doi: 10.1038/s41598-018-21899-5. Sci Rep. 2018. PMID: 29472601 Free PMC article.
-
Ion channel networks in the control of cerebral blood flow.J Cereb Blood Flow Metab. 2016 Mar;36(3):492-512. doi: 10.1177/0271678X15616138. Epub 2015 Nov 9. J Cereb Blood Flow Metab. 2016. PMID: 26661232 Free PMC article. Review.
-
Retinal Vessel Responses to Flicker Stimulation Are Impaired in Ca v 2.3-Deficient Mice-An in-vivo Evaluation Using Retinal Vessel Analysis (RVA).Front Neurol. 2021 Apr 13;12:659890. doi: 10.3389/fneur.2021.659890. eCollection 2021. Front Neurol. 2021. PMID: 33927686 Free PMC article.
-
Expression profile and protein translation of TMEM16A in murine smooth muscle.Am J Physiol Cell Physiol. 2010 Nov;299(5):C948-59. doi: 10.1152/ajpcell.00018.2010. Epub 2010 Aug 4. Am J Physiol Cell Physiol. 2010. PMID: 20686072 Free PMC article.
References
-
- Ball CJ, Wilson DP, Turner SP, Saint DA, Beltrame JF. Heterogeneity of L- and T-channels in the vasculature: rationale for the efficacy of combined L- and T-blockade. Hypertension. 2009;53:654–660. - PubMed
-
- Baylis C, Qiu C, Engels K. Comparison of L-type and mixed L- and T-type calcium channel blockers on kidney injury caused by deoxycorticosterone-salt hypertension in rats. Am J Kidney Dis. 2001;38:1292–1297. - PubMed
-
- Beltrame JF, Turner SP, Leslie SL, Solomon P, Freedman SB, Horowitz JD. The angiographic and clinical benefits of mibefradil in the coronary slow flow phenomenon. J Am Coll Cardiol. 2004;44:57–62. - PubMed
-
- Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res. 2008;46:138–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

