Telomeres: protecting chromosomes against genome instability
- PMID: 20125188
- PMCID: PMC2842081
- DOI: 10.1038/nrm2848
Telomeres: protecting chromosomes against genome instability
Abstract
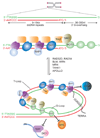
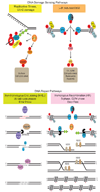
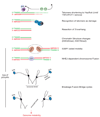
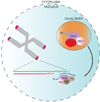
The natural ends of linear chromosomes require unique genetic and structural adaptations to facilitate the protection of genetic material. This is achieved by the sequestration of the telomeric sequence into a protective nucleoprotein cap that masks the ends from constitutive exposure to the DNA damage response machinery. When telomeres are unmasked, genome instability arises. Balancing capping requirements with telomere replication and the enzymatic processing steps that are obligatory for telomere function is a complex problem. Telomeric proteins and their interacting factors create an environment at chromosome ends that inhibits DNA repair; however, the repair machinery is essential for proper telomere function.
Figures

References
-
- Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. - PubMed
-
- Watson JD. Origin of concatemeric T7 DNA. Nat. New Biol. 1972;239:197–201. - PubMed
-
-
Griffith JD, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. This mansucript details the finding that telomeres can form a looped structure that could distinguish natural chromosome ends form DNA breaks.
-
-
- Oganesian L, Bryan TM. Physiological relevance of telomeric G-quadruplex formation: a potential drug target. Bioessays. 2007;29:155–165. - PubMed
-
- Rhodes D, Giraldo R. Telomere structure and function. Curr Opin Struct Biol. 1995;5:311–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

