Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner
- PMID: 20126258
- PMCID: PMC2811152
- DOI: 10.1371/journal.pbio.1000296
Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner
Expression of concern in
-
Expression of Concern: Global Regulator SATB1 Recruits β-Catenin and Regulates TH2 Differentiation in Wnt-Dependent Manner.PLoS Biol. 2022 Nov 23;20(11):e3001908. doi: 10.1371/journal.pbio.3001908. eCollection 2022 Nov. PLoS Biol. 2022. PMID: 36417696 Free PMC article. No abstract available.
Abstract
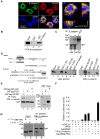
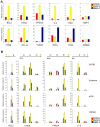
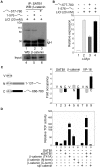
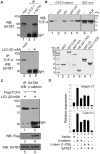
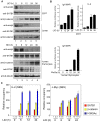
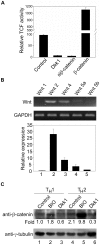
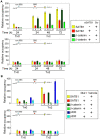
In vertebrates, the conserved Wnt signalling cascade promotes the stabilization and nuclear accumulation of beta-catenin, which then associates with the lymphoid enhancer factor/T cell factor proteins (LEF/TCFs) to activate target genes. Wnt/beta -catenin signalling is essential for T cell development and differentiation. Here we show that special AT-rich binding protein 1 (SATB1), the T lineage-enriched chromatin organizer and global regulator, interacts with beta-catenin and recruits it to SATB1's genomic binding sites. Gene expression profiling revealed that the genes repressed by SATB1 are upregulated upon Wnt signalling. Competition between SATB1 and TCF affects the transcription of TCF-regulated genes upon beta-catenin signalling. GATA-3 is a T helper type 2 (T(H)2) specific transcription factor that regulates production of T(H)2 cytokines and functions as T(H)2 lineage determinant. SATB1 positively regulated GATA-3 and siRNA-mediated knockdown of SATB1 downregulated GATA-3 expression in differentiating human CD4(+) T cells, suggesting that SATB1 influences T(H)2 lineage commitment by reprogramming gene expression. In the presence of Dickkopf 1 (Dkk1), an inhibitor of Wnt signalling, GATA-3 is downregulated and the expression of signature T(H)2 cytokines such as IL-4, IL-10, and IL-13 is reduced, indicating that Wnt signalling is essential for T(H)2 differentiation. Knockdown of beta-catenin also produced similar results, confirming the role of Wnt/beta-catenin signalling in T(H)2 differentiation. Furthermore, chromatin immunoprecipitation analysis revealed that SATB1 recruits beta-catenin and p300 acetyltransferase on GATA-3 promoter in differentiating T(H)2 cells in a Wnt-dependent manner. SATB1 coordinates T(H)2 lineage commitment by reprogramming gene expression. The SATB1:beta-catenin complex activates a number of SATB1 regulated genes, and hence this study has potential to find novel Wnt responsive genes. These results demonstrate that SATB1 orchestrates T(H)2 lineage commitment by mediating Wnt/beta-catenin signalling. This report identifies a new global transcription factor involved in beta-catenin signalling that may play a major role in dictating the functional outcomes of this signalling pathway during development, differentiation, and tumorigenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Comment in
-
Editorial Note: Global Regulator SATB1 Recruits β-Catenin and Regulates TH2 Differentiation in Wnt-Dependent Manner.PLoS Biol. 2025 Nov 20;23(11):e3003507. doi: 10.1371/journal.pbio.3003507. eCollection 2025 Nov. PLoS Biol. 2025. PMID: 41264553 Free PMC article. No abstract available.
References
-
- Logan C. Y, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
-
- Staal F. J, Clevers H. C. Wnt signaling in the thymus. Curr Opin Immunol. 2003;15:204–208. - PubMed
-
- Itoh K, Krupnik V. E, Sokol S. Y. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol. 1998;8:591–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

