Rufinamide: a novel broad-spectrum antiepileptic drug
- PMID: 20126329
- PMCID: PMC2812713
- DOI: 10.1111/j.1535-7511.2009.01336.x
Rufinamide: a novel broad-spectrum antiepileptic drug
Abstract
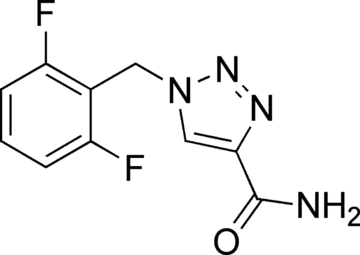
The last 20 years have witnessed a tremendous explosion in the number of antiepileptic drugs (AEDs) as well as the introduction of AEDS developed for specific epilepsy syndromes. The study of the efficacy and side effect profile of AEDs for unique epilepsy syndromes has allowed neurologists to utilize evidence-based medicine when treating patients. In late 2008, the Food and Drug Administration approved rufinamide for adjunctive use in the treatment of seizures associated with Lennox-Gastaut syndrome. This unique chemical compound is also the first new AED to reach the market in the United States having a pediatric indication prior to approval for adults. Rufinamide appears to have a broad spectrum of efficacy, is well tolerated, and may be rapidly initiated--properties that will likely extend its use outside of Lennox-Gastaut syndrome.
Figures
References
-
- White HS, Franklin MR, Kupferberg HJ, Schmutz M, Stables JP, Wolf HH. The anticonvulsant profile of rufinamide (CGP33101) in rodent seizure models. Epilepsia. 2008;49:1213–1220. - PubMed
-
- Perucca E, Cloyd J, Critchley D, Fuseau E. Rufinamide: Clinical Pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia. 2008;49:1123–1141. - PubMed
-
- Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, Leppick IE, Tomson T, Perucca E. Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49:1239–1276. - PubMed
-
- Perucca E. Is there a role for therapeutic drug monitoring of new anticonvulsants? Clin Pharmacokinet. 2000;38:191–204. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

