CAML does not modulate tetherin-mediated restriction of HIV-1 particle release
- PMID: 20126395
- PMCID: PMC2814849
- DOI: 10.1371/journal.pone.0009005
CAML does not modulate tetherin-mediated restriction of HIV-1 particle release
Abstract
Background: Tetherin/BST-2 is a recently-identified potent restriction factor in human cells that restricts HIV particle release following particle formation and budding at the plasma membrane. Vpu counteracts tetherin's restriction of particle release in a manner that has not yet been fully defined. We recently identified calcium-modulating cyclophilin ligand (CAML) as a Vpu-interacting protein that also restricts particle release. We hypothesized that CAML may act to enhance tetherin-mediated restriction of particle release and thereby explain how two distinct factors could be responsible for Vpu-responsive restriction.
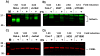
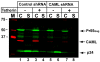
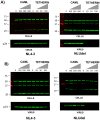
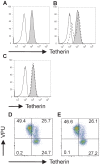
Methodology/principal findings: Endogenous levels of tetherin in human cells correlated well with their restriction pattern and responsiveness to Vpu, while levels of cellular CAML protein did not. Tetherin but not CAML was inducible by interferon in a wide variety of human cells. Stable depletion of human CAML in restrictive HeLa cells had no effect on cell surface levels of tetherin, and failed to relieve tetherin-mediated restriction. Stable depletion of tetherin from HeLa cells, in contrast, rendered HeLa cells permissive and Vpu-unresponsive. Tetherin but not CAML expression in permissive human cells rendered them restrictive and Vpu responsive. Depletion of CAML had no influence on cell surface levels of tetherin.
Conclusions/significance: We conclude that tetherin restricts particle release and does not require CAML for this effect. Furthermore, these results do not support a major role for CAML in restricting HIV particle release in human cells.
Conflict of interest statement
Figures


Similar articles
-
Effect of calcium-modulating cyclophilin ligand on human immunodeficiency virus type 1 particle release and cell surface expression of tetherin.J Virol. 2009 Dec;83(24):13032-6. doi: 10.1128/JVI.01786-09. Epub 2009 Sep 30. J Virol. 2009. PMID: 19793820 Free PMC article.
-
Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion.PLoS Pathog. 2009 May;5(5):e1000443. doi: 10.1371/journal.ppat.1000443. Epub 2009 May 22. PLoS Pathog. 2009. PMID: 19461879 Free PMC article.
-
Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells.J Virol. 2010 Dec;84(23):12185-99. doi: 10.1128/JVI.01447-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861257 Free PMC article.
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
-
Transmembrane interactions of HIV-1 Vpu and tetherin.Curr HIV Res. 2012 Jun;10(4):292-7. doi: 10.2174/157016212800792450. Curr HIV Res. 2012. PMID: 22524177 Review.
Cited by
-
BST-2 mediated restriction of simian-human immunodeficiency virus.Virology. 2010 Oct 25;406(2):312-21. doi: 10.1016/j.virol.2010.07.021. Epub 2010 Aug 13. Virology. 2010. PMID: 20708210 Free PMC article.
-
Unravelling the Immunomodulatory Effects of Viral Ion Channels, towards the Treatment of Disease.Viruses. 2021 Oct 27;13(11):2165. doi: 10.3390/v13112165. Viruses. 2021. PMID: 34834972 Free PMC article. Review.
-
ROCK1 and LIM kinase modulate retrovirus particle release and cell-cell transmission events.J Virol. 2014 Jun;88(12):6906-21. doi: 10.1128/JVI.00023-14. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696479 Free PMC article.
-
Ebola Virus Requires Phosphatidylserine Scrambling Activity for Efficient Budding and Optimal Infectivity.J Virol. 2021 Sep 27;95(20):e0116521. doi: 10.1128/JVI.01165-21. Epub 2021 Jul 28. J Virol. 2021. PMID: 34319156 Free PMC article.
References
-
- Arrigo SJ, Chen IS. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Genes Dev. 1991;5:808–819. - PubMed
-
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, et al. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

