Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis
- PMID: 20126399
- PMCID: PMC2814853
- DOI: 10.1371/journal.pone.0008999
Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis
Abstract
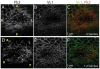
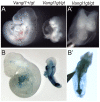
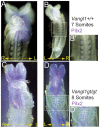
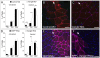
Left-right asymmetry in vertebrates is initiated in an early embryonic structure called the ventral node in human and mouse, and the gastrocoel roof plate (GRP) in the frog. Within these structures, each epithelial cell bears a single motile cilium, and the concerted beating of these cilia produces a leftward fluid flow that is required to initiate left-right asymmetric gene expression. The leftward fluid flow is thought to result from the posterior tilt of the cilia, which protrude from near the posterior portion of each cell's apical surface. The cells, therefore, display a morphological planar polarization. Planar cell polarity (PCP) is manifested as the coordinated, polarized orientation of cells within epithelial sheets, or as directional cell migration and intercalation during convergent extension. A set of evolutionarily conserved proteins regulates PCP. Here, we provide evidence that vertebrate PCP proteins regulate planar polarity in the mouse ventral node and in the Xenopus gastrocoel roof plate. Asymmetric anterior localization of VANGL1 and PRICKLE2 (PK2) in mouse ventral node cells indicates that these cells are planar polarized by a conserved molecular mechanism. A weakly penetrant Vangl1 mutant phenotype suggests that compromised Vangl1 function may be associated with left-right laterality defects. Stronger functional evidence comes from the Xenopus GRP, where we show that perturbation of VANGL2 protein function disrupts the posterior localization of motile cilia that is required for leftward fluid flow, and causes aberrant expression of the left side-specific gene Nodal. The observation of anterior-posterior PCP in the mouse and in Xenopus embryonic organizers reflects a strong evolutionary conservation of this mechanism that is important for body plan determination.
Conflict of interest statement
Figures

References
-
- Basu B, Brueckner M. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–174. - PubMed
-
- Shiratori H, Hamada H. The left-right axis in the mouse: from origin to morphology. Development. 2006;133:2095–2104. - PubMed
-
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. - PubMed
-
- Tabin CJ. The key to left-right asymmetry. Cell. 2006;127:27–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

