A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells
- PMID: 20126401
- PMCID: PMC2814855
- DOI: 10.1371/journal.pone.0009009
A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells
Abstract
Background: Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system (CNS). One potential therapeutic strategy for MS is to induce regulatory cells that mediate immunological tolerance. Probiotics, including lactobacilli, are known to induce immunomodulatory activity with promising effects in inflammatory diseases. We tested the potential of various strains of lactobacilli for suppression of experimental autoimmune encephalomyelitis (EAE), an animal model of MS.
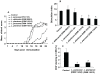
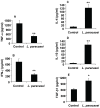
Methodology/principal findings: The preventive effects of five daily-administered strains of lactobacilli were investigated in mice developing EAE. After a primary screening, three Lactobacillus strains, L. paracasei DSM 13434, L. plantarum DSM 15312 and DSM 15313 that reduced inflammation in CNS and autoreactive T cell responses were chosen. L. paracasei and L. plantarum DSM 15312 induced CD4(+)CD25(+)Foxp3(+) regulatory T cells (Tregs) in mesenteric lymph nodes (MLNs) and enhanced production of serum TGF-beta1, while L. plantarum DSM 15313 increased serum IL-27 levels. Further screening of the chosen strains showed that each monostrain probiotic failed to be therapeutic in diseased mice, while a mixture of the three lactobacilli strains suppressed the progression and reversed the clinical and histological signs of EAE. The suppressive activity correlated with attenuation of pro-inflammatory Th1 and Th17 cytokines followed by IL-10 induction in MLNs, spleen and blood. Additional adoptive transfer studies demonstrated that IL-10 producing CD4(+)CD25(+) Tregs are involved in the suppressive effect induced by the lactobacilli mixture.
Conclusions/significance: Our data provide evidence showing that the therapeutic effect of the chosen mixture of probiotic lactobacilli was associated with induction of transferable tolerogenic Tregs in MLNs, but also in the periphery and the CNS, mediated through an IL-10-dependent mechanism. Our findings indicate a therapeutic potential of oral administration of a combination of probiotics and provide a more complete understanding of the host-commensal interactions that contribute to beneficial effects in autoimmune diseases.
Conflict of interest statement
Figures


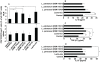
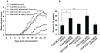
References
-
- Keegan BM, Noseworthy JH. MULTIPLE SCLEROSIS. Annual Review of Medicine. 2002;53:285–302. - PubMed
-
- Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung HP. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol. 2006;2:201–211. - PubMed
-
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. - PubMed
-
- Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

