The high affinity IgE receptor Fc epsilonRI is expressed by human intestinal epithelial cells
- PMID: 20126404
- PMCID: PMC2814858
- DOI: 10.1371/journal.pone.0009023
The high affinity IgE receptor Fc epsilonRI is expressed by human intestinal epithelial cells
Abstract
Background: IgE antibodies play a paramount role in the pathogenesis of various intestinal disorders. To gain insights in IgE-mediated pathophysiology of the gut, we investigated the expression of the high affinity IgE receptor Fc epsilonRI in human intestinal epithelium.
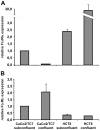
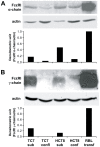
Methodology/principal findings: Fc epsilonRI alpha-chain, as detected by immunohistochemistry, was positive in epithelial cells for eight of eleven (8/11) specimens from colon cancer patients and 5/11 patients with inflammation of the enteric mucosa. The Fc epsilonRIalpha positive epithelial cells co-expressed Fc epsilonRIgamma, whereas with one exception, none of the samples was positive for the beta-chain in the epithelial layer. The functionality of Fc epsilonRI was confirmed in situ by human IgE binding. In experiments with human intestinal tumor cell lines, subconfluent Caco-2/TC7 and HCT-8 cells were found to express the alpha- and gamma-chains of Fc epsilonRI and to bind IgE, whereas confluent cells were negative for gamma-chains.
Conclusions/significance: Our data provide the first evidence that the components of a functional Fc epsilonRI are in vitro expressed by the human intestinal epithelial cells depending on differentiation and, more importantly, in situ in epithelia of patients with colon cancer or gastrointestinal inflammations. Thus, a contribution of Fc epsilonRI either to immunosurveillance or pathophysiology of the intestinal epithelium is suggested.
Conflict of interest statement
Figures





References
-
- Brandtzaeg P. History of oral tolerance and mucosal immunity. Ann N Y Acad Sci. 1996;778:1–27. - PubMed
-
- Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. - PubMed
-
- Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. - PubMed
-
- Huber A, Genser D, Spitzauer S, Scheiner O, Jensen-Jarolim E. IgE/anti-IgE immune complexes in sera from patients with Crohn's disease do not contain food-specific IgE. Int Arch Allergy Immunol. 1998;115:67–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

