A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines
- PMID: 20126405
- PMCID: PMC2814859
- DOI: 10.1371/journal.pone.0009001
A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines
Abstract
Background: The cytidine nucleoside analogs azacitidine (AZA) and decitabine (DAC) are used for the treatment of patients with myelodysplastic syndromes and acute myeloid leukemia (AML). Few non-clinical studies have directly compared the mechanisms of action of these agents in a head-to-head fashion, and the agents are often viewed as mechanistically similar DNA hypomethylating agents. To better understand the similarities and differences in mechanisms of these drugs, we compared their in vitro effects on several end points in human AML cell lines.
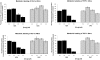
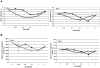
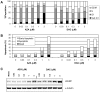
Methodology/principal findings: Both drugs effected DNA methyltransferase 1 depletion, DNA hypomethylation, and DNA damage induction, with DAC showing equivalent activity at concentrations 2- to 10-fold lower than AZA. At concentrations above 1 microM, AZA had a greater effect than DAC on reducing cell viability. Both drugs increased the sub-G1 fraction and apoptosis markers, with AZA decreasing all cell cycle phases and DAC causing an increase in G2-M. Total protein synthesis was reduced only by AZA, and drug-modulated gene expression profiles were largely non-overlapping.
Conclusions/significance: These data demonstrate shared mechanisms of action of AZA and DAC on DNA-mediated markers of activity, but distinctly different effects in their actions on cell viability, protein synthesis, cell cycle, and gene expression. The differential effects of AZA may be mediated by RNA incorporation, as the distribution of AZA in nucleic acid of KG-1a cells was 65:35, RNA:DNA.
Conflict of interest statement
Figures




References
-
- Cataldo VD, Cortes J, Quintás-Cardama A. Azacitidine for the treatment of myelodysplastic syndrome. Expert Rev Anticancer Ther. 2009;9:875–884. - PubMed
-
- Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. - PubMed
-
- Li LH, Olin EJ, Buskirk HH, Reineke LM. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970;30:2760–2769. - PubMed
-
- Glover AB, Leyland-Jones B. Biochemistry of azacitidine: a review. Cancer Treat Rep. 1987;71:959–964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

