Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially
- PMID: 20126406
- PMCID: PMC2814860
- DOI: 10.1371/journal.pone.0009016
Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially
Abstract
Background: Wharton's jelly derived stem cells (WJMSCs) are gaining attention as a possible clinical alternative to bone marrow derived mesenchymal stem cells (BMMSCs) owing to better accessibility, higher expansion potential and low immunogenicity. Usage of allogenic mesenchymal stem cells (MSC) could be permissible in vivo only if they retain their immune properties in an inflammatory setting. Thus the focus of this study is to understand and compare the immune properties of BMMSCs and WJMSCs primed with key pro-inflammatory cytokines, Interferon-gamma (IFNgamma) and Tumor Necrosis Factor-alpha (TNFalpha).
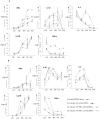
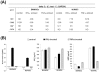
Methodology/principal findings: Initially the effect of priming on MSC mediated suppression of alloantigen and mitogen induced lymphoproliferation was evaluated in vitro. Treatment with IFNgamma or TNFalpha, did not ablate the immune-suppression caused by both the MSCs. Extent of immune-suppression was more with WJMSCs than BMMSCs in both the cases. Surprisingly, priming BMMSCs enhanced suppression of mitogen driven lymphoproliferation only; whereas IFNgamma primed WJMSCs were better suppressors of MLRs. Further, kinetic analysis of cytokine profiles in co-cultures of primed/unprimed MSCs and Phytohematoagglutinin (PHA) activated lymphocytes was evaluated. Results indicated a decrease in levels of pro-inflammatory cytokines. Interestingly, a change in kinetics and thresholds of Interleukin-2 (IL-2) secretion was observed only with BMMSCs. Analysis of activation markers on PHA-stimulated lymphocytes indicated different expression patterns in co-cultures of primed/unprimed WJMSCs and BMMSCs. Strikingly, co-culture with WJMSCs resulted in an early activation of a negative co-stimulatory molecule, CTLA4, which was not evident with BMMSCs. A screen for immune suppressive factors in primed/unprimed WJMSCs and BMMSCs indicated inherent differences in IFNgamma inducible Indoleamine 2, 3-dioxygenase (IDO) activity, Hepatocyte growth factor (HGF) and Prostaglandin E-2 (PGE2) levels which could possibly influence the mechanism of immune-modulation.
Conclusion/significance: This study demonstrates that inflammation affects the immune properties of MSCs distinctly. Importantly different tissue derived MSCs could utilize unique mechanisms of immune-modulation.
Conflict of interest statement
Figures



Similar articles
-
Decoding Cytokine Dynamics: Wharton's Jelly Stromal Cells and Chondro-Differentiates in PHA-Stimulated Co-Culture.Cells. 2025 Jan 23;14(3):174. doi: 10.3390/cells14030174. Cells. 2025. PMID: 39936966 Free PMC article.
-
Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis.J Tissue Eng Regen Med. 2019 Oct;13(10):1792-1804. doi: 10.1002/term.2930. Epub 2019 Jul 25. J Tissue Eng Regen Med. 2019. PMID: 31293088
-
Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament.J Cell Mol Med. 2014 Feb;18(2):344-54. doi: 10.1111/jcmm.12192. Epub 2014 Jan 3. J Cell Mol Med. 2014. PMID: 24393246 Free PMC article.
-
Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells.Regen Med. 2011 Jan;6(1):95-109. doi: 10.2217/rme.10.98. Regen Med. 2011. PMID: 21175290 Free PMC article. Review.
-
Bone marrow mesenchymal stem cells: fat on and blast off by FGF21.Int J Biochem Cell Biol. 2013 Mar;45(3):546-9. doi: 10.1016/j.biocel.2012.12.014. Epub 2012 Dec 25. Int J Biochem Cell Biol. 2013. PMID: 23270727 Free PMC article. Review.
Cited by
-
Establishment of a Novel Fetal Growth Restriction Model and Development of a Stem-Cell Therapy Using Umbilical Cord-Derived Mesenchymal Stromal Cells.Front Cell Neurosci. 2020 Jul 28;14:212. doi: 10.3389/fncel.2020.00212. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32848614 Free PMC article.
-
Immune therapeutic potential of stem cells from human supernumerary teeth.J Dent Res. 2013 Jul;92(7):609-15. doi: 10.1177/0022034513490732. Epub 2013 May 22. J Dent Res. 2013. PMID: 23697344 Free PMC article.
-
Immunomodulatory Role of Mesenchymal Stem Cell Therapy in Vascularized Composite Allotransplantation.J Transplant. 2016;2016:6951693. doi: 10.1155/2016/6951693. Epub 2016 Oct 16. J Transplant. 2016. PMID: 27822384 Free PMC article. Review.
-
Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury.Front Immunol. 2022 Dec 1;13:1014013. doi: 10.3389/fimmu.2022.1014013. eCollection 2022. Front Immunol. 2022. PMID: 36532022 Free PMC article.
-
Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: A clinical pilot study.PLoS One. 2019 Aug 29;14(8):e0221317. doi: 10.1371/journal.pone.0221317. eCollection 2019. PLoS One. 2019. PMID: 31465445 Free PMC article.
References
-
- Friedenstein AJ, Chailakhyan RK, Latsinik NV Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. - PubMed
-
- Phinney DG, Prockop DJ. Concise Review: Mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair - current views. Stem Cells. 2007;25(11):2896–902. - PubMed
-
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise Review: Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

