Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines
- PMID: 20126449
- PMCID: PMC2813279
- DOI: 10.1371/journal.ppat.1000745
Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines
Abstract
The recent 2009 pandemic H1N1 virus infection in humans has resulted in nearly 5,000 deaths worldwide. Early epidemiological findings indicated a low level of infection in the older population (>65 years) with the pandemic virus, and a greater susceptibility in people younger than 35 years of age, a phenomenon correlated with the presence of cross-reactive immunity in the older population. It is unclear what virus(es) might be responsible for this apparent cross-protection against the 2009 pandemic H1N1 virus. We describe a mouse lethal challenge model for the 2009 pandemic H1N1 strain, used together with a panel of inactivated H1N1 virus vaccines and hemagglutinin (HA) monoclonal antibodies to dissect the possible humoral antigenic determinants of pre-existing immunity against this virus in the human population. By hemagglutinination inhibition (HI) assays and vaccination/challenge studies, we demonstrate that the 2009 pandemic H1N1 virus is antigenically similar to human H1N1 viruses that circulated from 1918-1943 and to classical swine H1N1 viruses. Antibodies elicited against 1918-like or classical swine H1N1 vaccines completely protect C57B/6 mice from lethal challenge with the influenza A/Netherlands/602/2009 virus isolate. In contrast, contemporary H1N1 vaccines afforded only partial protection. Passive immunization with cross-reactive monoclonal antibodies (mAbs) raised against either 1918 or A/California/04/2009 HA proteins offered full protection from death. Analysis of mAb antibody escape mutants, generated by selection of 2009 H1N1 virus with these mAbs, indicate that antigenic site Sa is one of the conserved cross-protective epitopes. Our findings in mice agree with serological data showing high prevalence of 2009 H1N1 cross-reactive antibodies only in the older population, indicating that prior infection with 1918-like viruses or vaccination against the 1976 swine H1N1 virus in the USA are likely to provide protection against the 2009 pandemic H1N1 virus. This data provides a mechanistic basis for the protection seen in the older population, and emphasizes a rationale for including vaccination of the younger, naïve population. Our results also support the notion that pigs can act as an animal reservoir where influenza virus HAs become antigenically frozen for long periods of time, facilitating the generation of human pandemic viruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
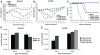

References
-
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. - PubMed
-
- Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Howley DMKPM, editor. Fields virology. 5th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1647–1689.
-
- Cox NJ, Neumann G, Donis RO, Kawaoka Y. Orthomyxoviruses: influenza. In: Mahy BWJ, ter Meulen V, editors. Topley and Wilson's microbiology and microbial infections. London: Hodder Arnold Press; 2005. pp. 634–698.
-
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

