Evaluation of skeletal and cardiac muscle function after chronic administration of thymosin beta-4 in the dystrophin deficient mouse
- PMID: 20126456
- PMCID: PMC2813286
- DOI: 10.1371/journal.pone.0008976
Evaluation of skeletal and cardiac muscle function after chronic administration of thymosin beta-4 in the dystrophin deficient mouse
Abstract
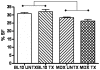
Thymosin beta-4 (Tbeta4) is a ubiquitous protein with many properties relating to cell proliferation and differentiation that promotes wound healing and modulates inflammatory mediators. We studied the effects of chronic administration of Tbeta4 on the skeletal and cardiac muscle of dystrophin deficient mdx mice, the mouse model of Duchenne muscular dystrophy. Female wild type (C57BL10/ScSnJ) and mdx mice, 8-10 weeks old, were treated with 150 microg of Tbeta4 twice a week for 6 months. To promote muscle pathology, mice were exercised for 30 minutes twice a week. Skeletal and cardiac muscle function were assessed via grip strength and high frequency echocardiography. Localization of Tbeta4 and amount of fibrosis were quantified using immunohistochemistry and Gomori's tri-chrome staining, respectively. Mdx mice treated with Tbeta4 showed a significant increase in skeletal muscle regenerating fibers compared to untreated mdx mice. Tbeta4 stained exclusively in the regenerating fibers of mdx mice. Although untreated mdx mice had significantly decreased skeletal muscle strength compared to untreated wild type, there were no significant improvements in mdx mice after treatment. Systolic cardiac function, measured as percent shortening fraction, was decreased in untreated mdx mice compared to untreated wild type and there was no significant difference after treatment in mdx mice. Skeletal and cardiac muscle fibrosis were also significantly increased in untreated mdx mice compared to wild type, but there was no significant improvement in treated mdx mice. In exercised dystrophin deficient mice, chronic administration of Tbeta4 increased the number of regenerating fibers in skeletal muscle and could have a potential role in treatment of skeletal muscle disease in Duchenne muscular dystrophy.
Conflict of interest statement
Figures
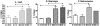


Similar articles
-
Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice.J Cardiovasc Pharmacol Ther. 2011 Mar;16(1):87-95. doi: 10.1177/1074248410381757. J Cardiovasc Pharmacol Ther. 2011. PMID: 21304057 Free PMC article.
-
Membrane sealant Poloxamer P188 protects against isoproterenol induced cardiomyopathy in dystrophin deficient mice.BMC Cardiovasc Disord. 2011 May 16;11:20. doi: 10.1186/1471-2261-11-20. BMC Cardiovasc Disord. 2011. PMID: 21575230 Free PMC article.
-
Tetrahydrobiopterin synthesis and metabolism is impaired in dystrophin-deficient mdx mice and humans.Acta Physiol (Oxf). 2021 Apr;231(4):e13627. doi: 10.1111/apha.13627. Epub 2021 Mar 8. Acta Physiol (Oxf). 2021. PMID: 33580591
-
Connexin-43 reduction prevents muscle defects in a mouse model of manifesting Duchenne muscular dystrophy female carriers.Sci Rep. 2020 Mar 30;10(1):5683. doi: 10.1038/s41598-020-62844-9. Sci Rep. 2020. PMID: 32231219 Free PMC article.
-
Deletion of Galgt2 (B4Galnt2) reduces muscle growth in response to acute injury and increases muscle inflammation and pathology in dystrophin-deficient mice.Am J Pathol. 2015 Oct;185(10):2668-84. doi: 10.1016/j.ajpath.2015.06.008. Am J Pathol. 2015. PMID: 26435413 Free PMC article.
Cited by
-
Transdifferentiation of Human Fibroblasts into Skeletal Muscle Cells: Optimization and Assembly into Engineered Tissue Constructs through Biological Ligands.Biology (Basel). 2021 Jun 16;10(6):539. doi: 10.3390/biology10060539. Biology (Basel). 2021. PMID: 34208436 Free PMC article.
-
Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice.J Cardiovasc Pharmacol Ther. 2011 Mar;16(1):87-95. doi: 10.1177/1074248410381757. J Cardiovasc Pharmacol Ther. 2011. PMID: 21304057 Free PMC article.
-
Gene co-expression network analysis provides novel insights into myostatin regulation at three different mouse developmental timepoints.PLoS One. 2015 Feb 19;10(2):e0117607. doi: 10.1371/journal.pone.0117607. eCollection 2015. PLoS One. 2015. PMID: 25695797 Free PMC article.
-
Long-term human IgG treatment improves heart and muscle function in a mouse model of Duchenne muscular dystrophy.J Cachexia Sarcopenia Muscle. 2020 Aug;11(4):1018-1031. doi: 10.1002/jcsm.12569. Epub 2020 May 20. J Cachexia Sarcopenia Muscle. 2020. PMID: 32436338 Free PMC article.
-
Exercise, healthy ageing, and the potential role of small extracellular vesicles.J Physiol. 2023 Nov;601(22):4937-4951. doi: 10.1113/JP282468. Epub 2022 Apr 29. J Physiol. 2023. PMID: 35388915 Free PMC article.
References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. - PubMed
-
- Anderson JE, Bressler BH, Ovalle WK. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988;9:499–515. - PubMed
-
- De Luca A, Pierno S, Liantonio A, Cetrone M, Camerino C, et al. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J Pharmacol Exp Ther. 2003;304:453–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

