Neuro-muscular differentiation of adult porcine skin derived stem cell-like cells
- PMID: 20126464
- PMCID: PMC2813294
- DOI: 10.1371/journal.pone.0008968
Neuro-muscular differentiation of adult porcine skin derived stem cell-like cells
Abstract
Background: Due to the genetic relationship to humans, porcine stem cells are a very important model system to investigate cell differentiation, associated cell signaling pathways, and cell fate. Porcine skin derived stem cells have been isolated from mid-gestation porcine fetus recently. To our knowledge, stem cells from the skin of the adult porcine organism have not been isolated until now. Hence, to our knowledge, we here describe the isolation, expansion, characterization and differentiation of multipotent porcine skin derived stem cell-like cells (pSSCs) from the adult porcine organism for the first time.
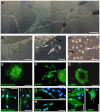
Methodology/principal findings: pSSCs had a spindle shaped morphology similar to mesenchymal stem cells (MSCs). They could be maintained proliferatively active in vitro for more than 120 days and were able to form colonies from single cells. pSSCs expressed Sox2 and Oct3/4, both transcription factors essential to the pluripotent and self-renewing phenotypes of embryonic stem cells, which recently gained attention due to their function in inducing pluripotent stem cells. Furthermore, the expression of the progenitor marker nestin, the somatic stem cell markers Bcrp1/ABCG2, Bmi1, and Stat3 was detected by reverse transcriptase-polymerase chain reaction (RT-PCR) in undifferentiated pSSCs. Flow cytometry revealed the expression of the MSC related proteins CD9, CD29, CD44 and CD105, but not CD90. After neuronal differentiation cells with a characteristic morphology of neuronal and smooth muscle-like cells were present in the cultures. Subsequent immunochemistry and flow cytometry revealed the down-regulation of nestin and the up-regulation of the neuron specific protein beta-III-tubulin and the astrocyte marker GFAP. Also, alpha-SMA expressing cells increased during differentiation suggesting the neuro-muscular differentiation of these skin derived cells. pSSCs could also be induced to differentiate into adipocyte-like cells when cultured under specific conditions.
Conclusions/significance: Adult porcine skin harbors a population of stem cell-like cells (pSSCs) that can be isolated via enzymatic digestion. These pSSCs show characteristic features of MSCs originated in other tissues and express the embryonic stem cell marker Oct3/4, Sox2, and Stat3. Furthermore, pSSCs have the potential to differentiate into cells from two different germ lines, the ectoderm (neurons, astrocytes) and the mesoderm (smooth muscle cells, adipocytes).
Conflict of interest statement
Figures
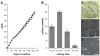



Similar articles
-
Characteristics of spermatogonial stem cells derived from neonatal porcine testis.Andrologia. 2015 Sep;47(7):765-78. doi: 10.1111/and.12327. Epub 2014 Sep 23. Andrologia. 2015. PMID: 25251288
-
[Biological characteristics of human umbilical cord-derived mesenchymal stem cells and their differentiation into neurocyte-like cells].Zhonghua Er Ke Za Zhi. 2006 Jul;44(7):513-7. Zhonghua Er Ke Za Zhi. 2006. PMID: 17044977 Chinese.
-
Isolation of retinal progenitor and stem cells from the porcine eye.Mol Vis. 2007 Jun 29;13:1045-57. Mol Vis. 2007. PMID: 17653049 Free PMC article.
-
[Lineage-switching by pluripotent cells derived from adults].J Soc Biol. 2001;195(1):39-46. J Soc Biol. 2001. PMID: 11530498 Review. French.
-
The Phoenix of stem cells: pluripotent cells in adult tissues and peripheral blood.Front Bioeng Biotechnol. 2024 Jul 30;12:1414156. doi: 10.3389/fbioe.2024.1414156. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39139297 Free PMC article. Review.
Cited by
-
Contribution to neural and mesodermal lineages by porcine skin-derived progenitors (SKPs) in vivo.Cell Cycle. 2010 May 15;9(10):2040-1. doi: 10.4161/cc.9.10.11688. Epub 2010 May 15. Cell Cycle. 2010. PMID: 20458191 Free PMC article. No abstract available.
-
Research Advancements in Porcine Derived Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(1):78-93. doi: 10.2174/1574888x10666150723145911. Curr Stem Cell Res Ther. 2016. PMID: 26201864 Free PMC article. Review.
-
Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source.J Vet Med Sci. 2016 Jul 1;78(6):987-95. doi: 10.1292/jvms.15-0596. Epub 2016 Feb 26. J Vet Med Sci. 2016. PMID: 26922917 Free PMC article.
-
Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas.Cell Prolif. 2011 Feb;44(1):19-32. doi: 10.1111/j.1365-2184.2010.00714.x. Cell Prolif. 2011. PMID: 21199007 Free PMC article.
-
Skin-derived stem cells as a source of primordial germ cell- and oocyte-like cells.Cell Death Dis. 2016 Nov 10;7(11):e2471. doi: 10.1038/cddis.2016.366. Cell Death Dis. 2016. PMID: 27831564 Free PMC article. Review.
References
-
- Dick I, Scott R. Pig ear skin as an in-vitro model for human skin permeability. The Journal of pharmacy and pharmacology. 1992;44:640–5. - PubMed
-
- Simon G, Maibach H. The Pig as an Experimental Animal Model of Percutaneous Permeation in Man: Qualitative and Quantitative Observations–An Overview. Skin Pharmacology and Applied Skin Physiology. 2000;13:229–234. - PubMed
-
- Jacobi U, Kaiser M, Toll R, Mangelsdorf S, Audring H, et al. Porcine ear skin: an in vitro model for human skin. Skin Research and Technology. 2007;13:19–24. - PubMed
-
- Strojek RM, Reed MA, Hoover JL, Wagner TE. A method for cultivating morphologically undifferentiated embryonic stem cells from porcine blastocysts. Theriogenology. 1990;33:901–913. - PubMed
-
- Piedrahita JA, Anderson GB, BonDurant RH. On the isolation of embryonic stem cells: Comparative behavior of murine, porcine and ovine embryos. Theriogenology. 1990;34:879–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

