Shorter exposures to harder X-rays trigger early apoptotic events in Xenopus laevis embryos
- PMID: 20126466
- PMCID: PMC2813296
- DOI: 10.1371/journal.pone.0008970
Shorter exposures to harder X-rays trigger early apoptotic events in Xenopus laevis embryos
Abstract
Background: A long-standing conventional view of radiation-induced apoptosis is that increased exposure results in augmented apoptosis in a biological system, with a threshold below which radiation doses do not cause any significant increase in cell death. The consequences of this belief impact the extent to which malignant diseases and non-malignant conditions are therapeutically treated and how radiation is used in combination with other therapies. Our research challenges the current dogma of dose-dependent induction of apoptosis and establishes a new parallel paradigm to the photoelectric effect in biological systems.
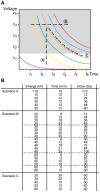
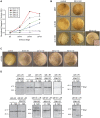
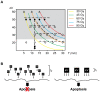
Methodology/principal findings: We explored how the energy of individual X-ray photons and exposure time, both factors that determine the total dose, influence the occurrence of cell death in early Xenopus embryo. Three different experimental scenarios were analyzed and morphological and biochemical hallmarks of apoptosis were evaluated. Initially, we examined cell death events in embryos exposed to increasing incident energies when the exposure time was preset. Then, we evaluated the embryo's response when the exposure time was augmented while the energy value remained constant. Lastly, we studied the incidence of apoptosis in embryos exposed to an equal total dose of radiation that resulted from increasing the incoming energy while lowering the exposure time.
Conclusions/significance: Overall, our data establish that the energy of the incident photon is a major contributor to the outcome of the biological system. In particular, for embryos exposed under identical conditions and delivered the same absorbed dose of radiation, the response is significantly increased when shorter bursts of more energetic photons are used. These results suggest that biological organisms display properties similar to the photoelectric effect in physical systems and provide new insights into how radiation-mediated apoptosis should be understood and utilized for therapeutic purposes.
Conflict of interest statement
Figures




Similar articles
-
Survival fraction and phenotype alterations of Xenopus laevis embryos at 3 Gy, 150 kV X-ray irradiation.Biochem Biophys Res Commun. 2016 Nov 25;480(4):580-585. doi: 10.1016/j.bbrc.2016.10.095. Epub 2016 Oct 26. Biochem Biophys Res Commun. 2016. PMID: 27793665
-
Effect of Photon Hormesis on Dose Responses to Alpha Particles in Zebrafish Embryos.Int J Mol Sci. 2017 Feb 11;18(2):385. doi: 10.3390/ijms18020385. Int J Mol Sci. 2017. PMID: 28208665 Free PMC article.
-
Ionizing radiation induces neurotoxicity in Xenopus laevis embryos through neuroactive ligand-receptor interaction pathway.Environ Res. 2024 Sep 1;256:119237. doi: 10.1016/j.envres.2024.119237. Epub 2024 May 27. Environ Res. 2024. PMID: 38810829
-
Responses to DNA damage in Xenopus: cell death or cell cycle arrest.Novartis Found Symp. 2001;237:221-30; discussion 230-4. doi: 10.1002/0470846666.ch17. Novartis Found Symp. 2001. PMID: 11444046 Review.
-
[Cement gland as the adhesion organ in Xenopus laevis embryos].Ontogenez. 2012 Jan-Feb;43(1):3-13. Ontogenez. 2012. PMID: 22567923 Review. Russian.
Cited by
-
On X-ray Sensitivity in Xenopus Embryogenesis.MicroPubl Biol. 2025 Apr 8;2025:10.17912/micropub.biology.001567. doi: 10.17912/micropub.biology.001567. eCollection 2025. MicroPubl Biol. 2025. PMID: 40270682 Free PMC article.
-
Realistic dosimetry for studies on biological responses to X-rays and γ-rays.J Radiat Res. 2017 Sep 1;58(5):729-736. doi: 10.1093/jrr/rrx019. J Radiat Res. 2017. PMID: 28444359 Free PMC article.
-
Biphasic and triphasic dose responses in zebrafish embryos to low-dose 150 kV X-rays with different levels of hardness.J Radiat Res. 2016 Jul;57(4):363-9. doi: 10.1093/jrr/rrw026. Epub 2016 Mar 6. J Radiat Res. 2016. PMID: 26951078 Free PMC article.
References
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. - PubMed
-
- Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–783. - PubMed
-
- Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. - PubMed
-
- Nijhawan D, Honarpour N, Wang X. Apoptosis in neural development and disease. Annu Rev Neurosci. 2000;23:73–87. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

