CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis
- PMID: 20126467
- PMCID: PMC2813297
- DOI: 10.1371/journal.pone.0008959
CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis
Abstract
Background: Although the etiology of idiopathic pulmonary fibrosis (IPF) remains perplexing, adaptive immune activation is evident among many afflicted patients. Repeated cycles of antigen-induced proliferation cause T-cells to lose surface expression of CD28, and we hypothesized this process might also occur in IPF.
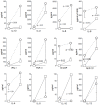
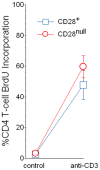
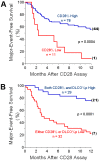
Methodology/principal findings: Peripheral blood CD4 T-cells from 89 IPF patients were analyzed by flow cytometry and cytokine multiplex assays, and correlated with clinical events. In comparison to autologous CD4(+)CD28(+)cells, the unusual CD4(+)CD28(null) lymphocytes seen in many IPF patients had discordant expressions of activation markers, more frequently produced cytotoxic mediators perforin (2.4+/-0.8% vs. 60.0+/-7.4%, p<0.0001) and granzyme B (4.5+/-2.8% vs.74.9+/-6.5%, p<0.0001), produced greater amounts of many pro-inflammatory cytokines, and less frequently expressed the regulatory T-cell marker FoxP3 (12.9+/-1.1% vs. 3.3+/-0.6% p<0.0001). Infiltration of CD4(+)CD28(null) T-cells in IPF lungs was confirmed by confocal microscopy. Interval changes of CD28 expression among subjects who had replicate studies were correlated with conterminous changes of their forced vital capacities (r(s) = 0.49, p = 0.012). Most importantly, one-year freedom from major adverse clinical events (either death or lung transplantation) was 56+/-6% among 78 IPF patients with CD4(+)CD28(+)/CD4(total)>or=82%, compared to 9+/-9% among those with more extensive CD28 down-regulation (CD4(+)CD28(+)/CD4(total)<82%) (p = 0.0004). The odds ratio for major adverse events among those with the most extensive CD28 down-regulation was 13.0, with 95% confidence intervals 1.6-111.1.
Conclusions/significance: Marked down-regulation of CD28 on circulating CD4 T-cells, a result of repeated antigen-driven proliferations, is associated with poor outcomes in IPF patients. The CD4(+)CD28(null) cells of these patients have potentially enhanced pathogenic characteristics, including increased productions of cytotoxic mediators and pro-inflammatory cytokines. These findings show proliferative T-cell responses to antigen(s) resulting in CD28 down-regulation are associated with progression and manifestations of IPF, and suggest assays of circulating CD4 T-cells may identify patients at greatest risk for clinical deterioration.
Conflict of interest statement
Figures




References
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, et al. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:406–430. - PubMed
-
- Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181:756–767. - PubMed
-
- Takahashi T, Wada I, Ohtsuka Y, Munakata M, Homm Y, et al. Autoantibody to alanyl-tRNA synthetase in patients with idiopathic pulmonary fibrosis. Respirology. 2007;12:642–653. - PubMed
-
- Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, et al. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol. 2006;67:284–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

