GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche
- PMID: 20126470
- PMCID: PMC2814350
- DOI: 10.1593/neo.91384
GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche
Abstract
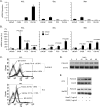
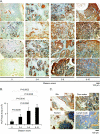
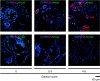
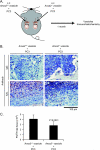
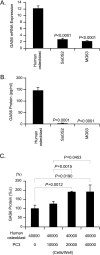
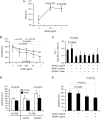
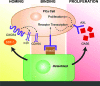
Our recent studies have shown that annexin II, expressed on the cell surface of osteoblasts, plays an important role in the adhesion of hematopoietic stem cells (HSCs) to the endosteal niche. Similarly, prostate cancer (PCa) cells express the annexin II receptor and seem to use the stem cell niche for homing to the bone marrow. The role of the niche is thought to be the induction and sustenance of HSC dormancy. If metastatic PCa cells occupy a similar or the same ecological niche as HSCs, then it is likely that the initial role of the HSC niche will be to induce dormancy in metastatic cells. In this study, we demonstrate that the binding of PCa to annexin II induces the expression of the growth arrest-specific 6 (GAS6) receptors AXL, Sky, and Mer, which, in the hematopoietic system, induce dormancy. In addition, GAS6 produced by osteoblasts prevents PCa proliferation and protects PCa from chemotherapy-induced apoptosis. Our results suggest that the activation of GAS6 receptors on PCa in the bone marrow environment may play a critical role as a molecular switch, establishing metastatic tumor cell dormancy.
Figures
Similar articles
-
Growth Arrest-Specific 6 (GAS6) Promotes Prostate Cancer Survival by G1 Arrest/S Phase Delay and Inhibition of Apoptosis During Chemotherapy in Bone Marrow.J Cell Biochem. 2016 Dec;117(12):2815-2824. doi: 10.1002/jcb.25582. Epub 2016 Sep 26. J Cell Biochem. 2016. PMID: 27153245 Free PMC article.
-
Endogenous GAS6 and Mer receptor signaling regulate prostate cancer stem cells in bone marrow.Oncotarget. 2016 May 3;7(18):25698-711. doi: 10.18632/oncotarget.8365. Oncotarget. 2016. PMID: 27028863 Free PMC article.
-
Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow.Sci Rep. 2016 Nov 7;6:36520. doi: 10.1038/srep36520. Sci Rep. 2016. PMID: 27819283 Free PMC article.
-
Biochemical Changes in the Niche Following Tumor Cell Invasion.J Cell Biochem. 2017 Aug;118(8):1956-1964. doi: 10.1002/jcb.25843. Epub 2017 Apr 18. J Cell Biochem. 2017. PMID: 27982511 Free PMC article. Review.
-
The prostate cancer bone marrow niche: more than just 'fertile soil'.Asian J Androl. 2012 May;14(3):423-7. doi: 10.1038/aja.2011.164. Epub 2012 Feb 27. Asian J Androl. 2012. PMID: 22367179 Free PMC article. Review.
Cited by
-
Extracellular Vesicle-Mediated Bone Remodeling and Bone Metastasis: Implications in Prostate Cancer.Subcell Biochem. 2021;97:297-361. doi: 10.1007/978-3-030-67171-6_12. Subcell Biochem. 2021. PMID: 33779922 Review.
-
Tumor dormancy in bone.Cancer Rep (Hoboken). 2020 Feb;3(1):e1156. doi: 10.1002/cnr2.1156. Epub 2019 Jan 29. Cancer Rep (Hoboken). 2020. PMID: 32632400 Free PMC article. Review.
-
The Role of TAM Receptors in Bone.Int J Mol Sci. 2023 Dec 23;25(1):233. doi: 10.3390/ijms25010233. Int J Mol Sci. 2023. PMID: 38203403 Free PMC article. Review.
-
Growth Arrest-Specific 6 (GAS6) Promotes Prostate Cancer Survival by G1 Arrest/S Phase Delay and Inhibition of Apoptosis During Chemotherapy in Bone Marrow.J Cell Biochem. 2016 Dec;117(12):2815-2824. doi: 10.1002/jcb.25582. Epub 2016 Sep 26. J Cell Biochem. 2016. PMID: 27153245 Free PMC article.
-
Gas6/Axl Signaling Pathway in the Tumor Immune Microenvironment.Cancers (Basel). 2020 Jul 9;12(7):1850. doi: 10.3390/cancers12071850. Cancers (Basel). 2020. PMID: 32660000 Free PMC article. Review.
References
-
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. - PubMed
-
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. - PubMed
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
