Constitutive expression of the alpha4 integrin correlates with tumorigenicity and lymph node metastasis of the B16 murine melanoma
- PMID: 20126475
- PMCID: PMC2814355
- DOI: 10.1593/neo.91604
Constitutive expression of the alpha4 integrin correlates with tumorigenicity and lymph node metastasis of the B16 murine melanoma
Abstract
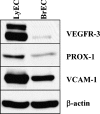
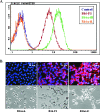
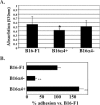
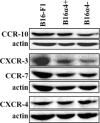
The lymphatic system plays a critical role in melanoma metastasis, and yet, virtually no information exists regarding the cellular and molecular mechanisms that take place between melanoma cells and the lymphatic vasculature. Here, we generated B16-F1 melanoma cells that expressed high (B16alpha(4)+) and negligible (B16alpha(4)-) levels of alpha(4) integrin to determine how the expression of alpha(4) integrins affects tumor cell interactions with lymphatic endothelial cells in vitro and how it impacts lymphatic metastasis in vivo. We found a direct correlation between alpha(4) integrin expression on B16-F1 melanoma cells and their ability to form adhesive interactions with monolayers of lymphatic endothelial cells. Adhesion of B16-F1 melanoma cells to lymphatic endothelial cells was mediated by the melanoma cell alpha(4) integrin binding to its counterreceptor, vascular cell adhesion molecule 1 (VCAM-1), that was constitutively expressed on the lymphatic endothelial cells. VCAM-1 was also expressed on the tumor-associated lymphatic vessels of B16-F1 and B16alpha(4)+ tumors growing in the subcutaneous space of C57BL/6J mice. B16-F1 tumors metastasized to lymph nodes in 30% of mice, whereas B16alpha(4)+ tumors generated lymph node metastases in 80% of mice. B16-F1 melanoma cells that were deficient in alpha(4) integrins (B16alpha(4)-) were nontumorigenic. Collectively, these data show that the alpha(4) integrin expressed by melanoma cells contributes to tumorigenesis and may also facilitate metastasis to regional lymph nodes by promoting stable adhesion of melanoma cells to the lymphatic vasculature.
Figures


References
-
- Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol. 1988;6:163–172. - PubMed
-
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, et al. New TNM melanoma staging system: linking biology and natural history to clinical outcomes. Semin Surg Oncol. 2003;21:43–52. - PubMed
-
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. - PubMed
-
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
