Mini drug pump for ophthalmic use
Abstract
Purpose: To evaluate the feasibility of developing a novel mini drug pump for ophthalmic use.
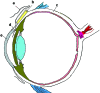
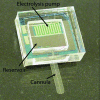
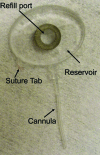
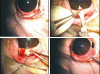
Methods: Using principles of microelectromechanical systems engineering, a mini drug pump was fabricated. The pumping mechanism is based on electrolysis, and the pump includes a drug refill port as well as a check valve to control drug delivery. Drug pumps were tested first on the benchtop and then after implantation in rabbits. For the latter, we implanted 4 elliptical (9.9 x 7.7 x 1.8 mm) non-electrically active pumps into 4 rabbits. The procedure is similar to implantation of a glaucoma seton. To determine the ability to refill and also the patency of the cannula, at intervals of 4 to 6 weeks after implantation, we accessed the drug reservoir with a transconjunctival needle and delivered approximately as low as 1 microL of trypan blue solution (0.06%) into the anterior chamber. Animals were followed up by slit-lamp examination, photography, and fluorescein angiography.
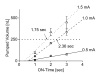
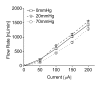
Results: Benchtop testing showed 2.0 microL/min delivery when using 0.4 mW of power for electrolysis. One-way valves showed reliable opening pressures of 470 mm Hg. All implanted devices refilled at 4- to 6-week intervals for 4 to 6 months. No infection was seen. No devices extruded. No filtering bleb formed over the implant.
Conclusions: A prototype ocular mini drug pump was built, implanted, and refilled. Such a platform needs more testing to determine the long-term biocompatibility of an electrically controlled implanted pump. Testing with various pharmacologic agents is needed to determine its ultimate potential for ophthalmic use.
Figures






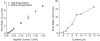


Similar articles
-
Mini drug pump for ophthalmic use.Curr Eye Res. 2010 Mar;35(3):192-201. doi: 10.3109/02713680903521936. Curr Eye Res. 2010. PMID: 20373877 Free PMC article.
-
A passive MEMS drug delivery pump for treatment of ocular diseases.Biomed Microdevices. 2009 Oct;11(5):959-70. doi: 10.1007/s10544-009-9313-9. Epub 2009 Apr 25. Biomed Microdevices. 2009. PMID: 19396548
-
Subcutaneous abdominal artificial tears pump-reservoir for severe dry eye.Orbit. 2003 Mar;22(1):29-40. doi: 10.1076/orbi.22.1.29.14012. Orbit. 2003. PMID: 12759865 Clinical Trial.
-
Mechanical pump dacryoreservoirs.Dev Ophthalmol. 2008;41:269-282. doi: 10.1159/000131095. Dev Ophthalmol. 2008. PMID: 18453775 Review.
-
Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses.Adv Colloid Interface Sci. 2016 Jul;233:139-154. doi: 10.1016/j.cis.2015.08.002. Epub 2015 Aug 14. Adv Colloid Interface Sci. 2016. PMID: 26318359 Review.
Cited by
-
Micro- and nano-fabricated implantable drug-delivery systems.Ther Deliv. 2012 Dec;3(12):1457-67. doi: 10.4155/tde.12.132. Ther Deliv. 2012. PMID: 23323562 Free PMC article. Review.
-
Microfluidics - The State-of-the-Art Technology for Pharmaceutical Application.Adv Pharm Bull. 2022 Aug;12(4):700-711. doi: 10.34172/apb.2022.073. Epub 2021 Sep 29. Adv Pharm Bull. 2022. PMID: 36415637 Free PMC article. Review.
-
Extended Duration Vascular Endothelial Growth Factor Inhibition in the Eye: Failures, Successes, and Future Possibilities.Pharmaceutics. 2018 Jan 27;10(1):21. doi: 10.3390/pharmaceutics10010021. Pharmaceutics. 2018. PMID: 29382038 Free PMC article. Review.
-
Nanomedicine and drug delivery to the retina: current status and implications for gene therapy.Naunyn Schmiedebergs Arch Pharmacol. 2022 Dec;395(12):1477-1507. doi: 10.1007/s00210-022-02287-3. Epub 2022 Sep 15. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 36107200 Free PMC article. Review.
-
Promising therapeutic drug delivery systems for glaucoma: a comprehensive review.Ther Adv Ophthalmol. 2020 Mar 13;12:2515841420905740. doi: 10.1177/2515841420905740. eCollection 2020 Jan-Dec. Ther Adv Ophthalmol. 2020. PMID: 32206746 Free PMC article. Review.
References
-
- Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000;41:961–964. - PubMed
-
- Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411–434. - PubMed
-
- Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007;18:235–239. - PubMed
-
- Shell JW. Ophthalmic drug delivery systems. Surv Ophthalmol. 1984;29:117–128. - PubMed
-
- Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol. 2000;27:558–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
