Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model
- PMID: 20126519
- PMCID: PMC2805432
- DOI: 10.3389/neuro.03.019.2009
Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model
Abstract
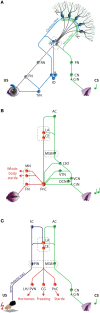
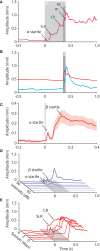
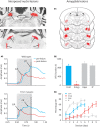
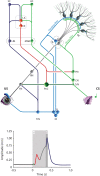
Over the past decade the advent of mouse transgenics has generated new perspectives on the study of cerebellar molecular mechanisms that are essential for eyeblink conditioning. However, it also appears that results from eyeblink conditioning experiments done in mice differ in some aspects from results previously obtained in other mammals. In this review article we will, based on studies using (cell-specific) mouse mutants and region-specific lesions, re-examine the general eyeblink behavior in mice and the neuro-anatomical circuits that might contribute to the different peaks in the conditioned eyeblink trace. We conclude that the learning process in mice has at least two stages: An early stage, which includes short-latency responses that are at least partly controlled by extracerebellar structures such as the amygdala, and a later stage, which is represented by well-timed conditioned responses that are mainly controlled by the pontocerebellar and olivocerebellar systems. We refer to this overall concept as the Amygdala-Cerebellum-Dynamic-Conditioning Model (ACDC model).
Keywords: ACDC model; amygdala; cerebellum; cued fear conditioning; eyeblink conditioning; mouse.
Figures
Similar articles
-
The neural circuitry and molecular mechanisms underlying delay and trace eyeblink conditioning in mice.Behav Brain Res. 2015 Feb 1;278:307-14. doi: 10.1016/j.bbr.2014.10.006. Epub 2014 Oct 17. Behav Brain Res. 2015. PMID: 25448430 Review.
-
Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats.J Neurosci. 2004 Mar 31;24(13):3242-50. doi: 10.1523/JNEUROSCI.5382-03.2004. J Neurosci. 2004. PMID: 15056703 Free PMC article.
-
Cerebellar-dependent expression of motor learning during eyeblink conditioning in head-fixed mice.J Neurosci. 2014 Nov 5;34(45):14845-53. doi: 10.1523/JNEUROSCI.2820-14.2014. J Neurosci. 2014. PMID: 25378152 Free PMC article.
-
Amygdala, deep cerebellar nuclei and red nucleus contribute to delay eyeblink conditioning in C57BL /6 mice.Eur J Neurosci. 2010 Nov;32(9):1537-51. doi: 10.1111/j.1460-9568.2010.07406.x. Epub 2010 Sep 30. Eur J Neurosci. 2010. PMID: 20880362
-
The involvement of the human cerebellum in eyeblink conditioning.Cerebellum. 2007;6(1):38-57. doi: 10.1080/14734220701225904. Cerebellum. 2007. PMID: 17366265 Review.
Cited by
-
Pre-ataxic loss of intrinsic plasticity and motor learning in a mouse model of SCA1.Brain. 2023 Jun 1;146(6):2332-2345. doi: 10.1093/brain/awac422. Brain. 2023. PMID: 36352508 Free PMC article.
-
Single-Unit Extracellular Recording from the Cerebellum During Eyeblink Conditioning in Head-Fixed Mice.Neuromethods. 2018;134:39-71. doi: 10.1007/978-1-4939-7549-5_3. Epub 2017 Dec 16. Neuromethods. 2018. PMID: 31156292 Free PMC article.
-
Cerebellar control of gait and interlimb coordination.Brain Struct Funct. 2015 Nov;220(6):3513-36. doi: 10.1007/s00429-014-0870-1. Epub 2014 Aug 20. Brain Struct Funct. 2015. PMID: 25139623 Free PMC article.
-
Locomotor activity modulates associative learning in mouse cerebellum.Nat Neurosci. 2018 May;21(5):725-735. doi: 10.1038/s41593-018-0129-x. Epub 2018 Apr 16. Nat Neurosci. 2018. PMID: 29662214 Free PMC article.
-
Spine loss in primary somatosensory cortex during trace eyeblink conditioning.J Neurosci. 2015 Mar 4;35(9):3772-81. doi: 10.1523/JNEUROSCI.2043-14.2015. J Neurosci. 2015. PMID: 25740508 Free PMC article.
References
-
- Albus J. S. (1971). A theory of cerebellar function. Math. Biosci. 10, 25–6110.1016/0025-5564(71)90051-4 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

