Armadillo motifs involved in vesicular transport
- PMID: 20126549
- PMCID: PMC2813876
- DOI: 10.1371/journal.pone.0008991
Armadillo motifs involved in vesicular transport
Abstract
Armadillo (ARM) repeat proteins function in various cellular processes including vesicular transport and membrane tethering. They contain an imperfect repeating sequence motif that forms a conserved three-dimensional structure. Recently, structural and functional insight into tethering mediated by the ARM-repeat protein p115 has been provided. Here we describe the p115 ARM-motifs for reasons of clarity and nomenclature and show that both sequence and structure are highly conserved among ARM-repeat proteins. We argue that there is no need to invoke repeat types other than ARM repeats for a proper description of the structure of the p115 globular head region. Additionally, we propose to define a new subfamily of ARM-like proteins and show lack of evidence that the ARM motifs found in p115 are present in other long coiled-coil tethering factors of the golgin family.
Conflict of interest statement
Figures

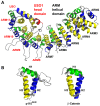
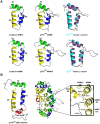
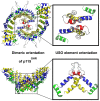

Similar articles
-
Unusual armadillo fold in the human general vesicular transport factor p115.PLoS One. 2009;4(2):e4656. doi: 10.1371/journal.pone.0004656. Epub 2009 Feb 27. PLoS One. 2009. PMID: 19247479 Free PMC article.
-
Structural and functional analysis of the globular head domain of p115 provides insight into membrane tethering.J Mol Biol. 2009 Aug 7;391(1):26-41. doi: 10.1016/j.jmb.2009.04.062. Epub 2009 May 4. J Mol Biol. 2009. PMID: 19414022 Free PMC article.
-
p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p.Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):522-6. doi: 10.1073/pnas.92.2.522. Proc Natl Acad Sci U S A. 1995. PMID: 7831323 Free PMC article.
-
SNAREs and traffic.Biochim Biophys Acta. 2005 Jul 10;1744(3):493-517. Biochim Biophys Acta. 2005. PMID: 16038056 Review.
-
SNAREs and traffic.Biochim Biophys Acta. 2005 Jun 30;1744(2):120-44. doi: 10.1016/j.bbamcr.2005.03.014. Epub 2005 Apr 21. Biochim Biophys Acta. 2005. Corrected and republished in: Biochim Biophys Acta. 2005 Jul 10;1744(3):493-517. PMID: 15893389 Corrected and republished. Review.
Cited by
-
The golgin coiled-coil proteins of the Golgi apparatus.Cold Spring Harb Perspect Biol. 2011 Jun 1;3(6):a005256. doi: 10.1101/cshperspect.a005256. Cold Spring Harb Perspect Biol. 2011. PMID: 21436057 Free PMC article. Review.
-
The LRRK2 signalling system.Cell Tissue Res. 2018 Jul;373(1):39-50. doi: 10.1007/s00441-017-2759-9. Epub 2018 Jan 8. Cell Tissue Res. 2018. PMID: 29308544 Free PMC article. Review.
-
ARMCX Family Gene Expression Analysis and Potential Prognostic Biomarkers for Prediction of Clinical Outcome in Patients with Gastric Carcinoma.Biomed Res Int. 2020 Jun 30;2020:3575038. doi: 10.1155/2020/3575038. eCollection 2020. Biomed Res Int. 2020. PMID: 32685472 Free PMC article.
-
Molecular characterization of an elicitor-responsive Armadillo repeat gene (GhARM) from cotton (Gossypium hirsutum).Mol Biol Rep. 2012 Aug;39(8):8513-23. doi: 10.1007/s11033-012-1706-9. Epub 2012 Jun 20. Mol Biol Rep. 2012. PMID: 22714909
-
Proteasomal Degradation of Zn-Dependent Hdacs: The E3-Ligases Implicated and the Designed Protacs That Enable Degradation.Molecules. 2021 Sep 15;26(18):5606. doi: 10.3390/molecules26185606. Molecules. 2021. PMID: 34577077 Free PMC article. Review.
References
-
- Riggleman B, Wieschaus E, Schedl P. Molecular analysis of the armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev. 1989;3:96–113. - PubMed
-
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. - PubMed
-
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296:1644–1646. - PubMed
-
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis - a look outside the nucleus. Science. 2000;287:1606–1609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

