The translation regulatory subunit eIF3f controls the kinase-dependent mTOR signaling required for muscle differentiation and hypertrophy in mouse
- PMID: 20126553
- PMCID: PMC2813880
- DOI: 10.1371/journal.pone.0008994
The translation regulatory subunit eIF3f controls the kinase-dependent mTOR signaling required for muscle differentiation and hypertrophy in mouse
Abstract
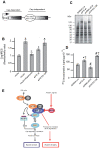
The mTORC1 pathway is required for both the terminal muscle differentiation and hypertrophy by controlling the mammalian translational machinery via phosphorylation of S6K1 and 4E-BP1. mTOR and S6K1 are connected by interacting with the eIF3 initiation complex. The regulatory subunit eIF3f plays a major role in muscle hypertrophy and is a key target that accounts for MAFbx function during atrophy. Here we present evidence that in MAFbx-induced atrophy the degradation of eIF3f suppresses S6K1 activation by mTOR, whereas an eIF3f mutant insensitive to MAFbx polyubiquitination maintained persistent phosphorylation of S6K1 and rpS6. During terminal muscle differentiation a conserved TOS motif in eIF3f connects mTOR/raptor complex, which phosphorylates S6K1 and regulates downstream effectors of mTOR and Cap-dependent translation initiation. Thus eIF3f plays a major role for proper activity of mTORC1 to regulate skeletal muscle size.
Conflict of interest statement
Figures






Similar articles
-
Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine.Am J Physiol Endocrinol Metab. 2010 Jun;298(6):E1283-94. doi: 10.1152/ajpendo.00676.2009. Epub 2010 Apr 13. Am J Physiol Endocrinol Metab. 2010. PMID: 20388826 Free PMC article.
-
TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function.Curr Biol. 2003 May 13;13(10):797-806. doi: 10.1016/s0960-9822(03)00329-4. Curr Biol. 2003. PMID: 12747827
-
Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size.Am J Physiol Cell Physiol. 2009 Jun;296(6):C1258-70. doi: 10.1152/ajpcell.00105.2009. Epub 2009 Apr 8. Am J Physiol Cell Physiol. 2009. PMID: 19357233
-
eIF3f: a central regulator of the antagonism atrophy/hypertrophy in skeletal muscle.Int J Biochem Cell Biol. 2013 Oct;45(10):2158-62. doi: 10.1016/j.biocel.2013.06.001. Epub 2013 Jun 13. Int J Biochem Cell Biol. 2013. PMID: 23769948 Free PMC article. Review.
-
The translational factor eIF3f: the ambivalent eIF3 subunit.Cell Mol Life Sci. 2013 Oct;70(19):3603-16. doi: 10.1007/s00018-013-1263-y. Epub 2013 Jan 25. Cell Mol Life Sci. 2013. PMID: 23354061 Free PMC article. Review.
Cited by
-
Role of IGF-I in follistatin-induced skeletal muscle hypertrophy.Am J Physiol Endocrinol Metab. 2015 Sep 15;309(6):E557-67. doi: 10.1152/ajpendo.00098.2015. Epub 2015 Jul 28. Am J Physiol Endocrinol Metab. 2015. PMID: 26219865 Free PMC article.
-
Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome.Int J Biochem Cell Biol. 2013 Oct;45(10):2121-9. doi: 10.1016/j.biocel.2013.04.023. Epub 2013 May 7. Int J Biochem Cell Biol. 2013. PMID: 23665154 Free PMC article. Review.
-
Estrogen receptor α promotes protein synthesis by fine-tuning the expression of the eukaryotic translation initiation factor 3 subunit f (eIF3f).J Biol Chem. 2019 Feb 15;294(7):2267-2278. doi: 10.1074/jbc.RA118.004383. Epub 2018 Dec 20. J Biol Chem. 2019. PMID: 30573685 Free PMC article.
-
mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong?Physiology (Bethesda). 2011 Apr;26(2):83-96. doi: 10.1152/physiol.00044.2010. Physiology (Bethesda). 2011. PMID: 21487027 Free PMC article. Review.
-
Enteral delivery of proteins enhances the expression of proteins involved in the cytoskeleton and protein biosynthesis in human duodenal mucosa.Am J Clin Nutr. 2015 Aug;102(2):359-67. doi: 10.3945/ajcn.114.104216. Epub 2015 Jun 24. Am J Clin Nutr. 2015. PMID: 26109581 Free PMC article. Clinical Trial.
References
-
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. - PubMed
-
- Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147–159. - PubMed
-
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. - PubMed
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
-
- Jacinto E, Loewith R, Schmidt A, Linn S, Rüegg MA, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

