A novel whole-cell biocatalyst with NAD+ regeneration for production of chiral chemicals
- PMID: 20126645
- PMCID: PMC2811184
- DOI: 10.1371/journal.pone.0008860
A novel whole-cell biocatalyst with NAD+ regeneration for production of chiral chemicals
Abstract
Background: The high costs of pyridine nucleotide cofactors have limited the applications of NAD(P)-dependent oxidoreductases on an industrial scale. Although NAD(P)H regeneration systems have been widely studied, NAD(P)(+) regeneration, which is required in reactions where the oxidized form of the cofactor is used, has been less well explored, particularly in whole-cell biocatalytic processes.
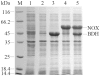
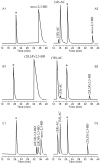
Methodology/principal findings: Simultaneous overexpression of an NAD(+) dependent enzyme and an NAD(+) regenerating enzyme (H(2)O producing NADH oxidase from Lactobacillus brevis) in a whole-cell biocatalyst was studied for application in the NAD(+)-dependent oxidation system. The whole-cell biocatalyst with (2R,3R)-2,3-butanediol dehydrogenase as the catalyzing enzyme was used to produce (3R)-acetoin, (3S)-acetoin and (2S,3S)-2,3-butanediol.
Conclusions/significance: A recombinant strain, in which an NAD(+) regeneration enzyme was coexpressed, displayed significantly higher biocatalytic efficiency in terms of the production of chiral acetoin and (2S,3S)-2,3-butanediol. The application of this coexpression system to the production of other chiral chemicals could be extended by using different NAD(P)-dependent dehydrogenases that require NAD(P)(+) for catalysis.
Conflict of interest statement
Figures



Similar articles
-
Efficient (3S)-Acetoin and (2S,3S)-2,3-Butanediol Production from meso-2,3-Butanediol Using Whole-Cell Biocatalysis.Molecules. 2018 Mar 19;23(3):691. doi: 10.3390/molecules23030691. Molecules. 2018. PMID: 29562693 Free PMC article.
-
Efficient whole-cell biocatalyst for acetoin production with NAD+ regeneration system through homologous co-expression of 2,3-butanediol dehydrogenase and NADH oxidase in engineered Bacillus subtilis.PLoS One. 2014 Jul 18;9(7):e102951. doi: 10.1371/journal.pone.0102951. eCollection 2014. PLoS One. 2014. PMID: 25036158 Free PMC article.
-
A new NAD(H)-dependent meso-2,3-butanediol dehydrogenase from an industrially potential strain Serratia marcescens H30.Appl Microbiol Biotechnol. 2014 Feb;98(3):1175-84. doi: 10.1007/s00253-013-4959-x. Epub 2013 May 12. Appl Microbiol Biotechnol. 2014. PMID: 23666479
-
Recent developments in pyridine nucleotide regeneration.Curr Opin Biotechnol. 2003 Aug;14(4):421-6. doi: 10.1016/s0958-1669(03)00094-6. Curr Opin Biotechnol. 2003. PMID: 12943852 Review.
-
Microbial alcohol dehydrogenases: recent developments and applications in asymmetric synthesis.Org Biomol Chem. 2024 Jan 3;22(2):228-251. doi: 10.1039/d3ob01447a. Org Biomol Chem. 2024. PMID: 38050738 Review.
Cited by
-
Engineering of cofactor regeneration enhances (2S,3S)-2,3-butanediol production from diacetyl.Sci Rep. 2013;3:2643. doi: 10.1038/srep02643. Sci Rep. 2013. PMID: 24025762 Free PMC article.
-
Biobutanol production in a Clostridium acetobutylicum biofilm reactor integrated with simultaneous product recovery by adsorption.Biotechnol Biofuels. 2014 Jan 8;7(1):5. doi: 10.1186/1754-6834-7-5. Biotechnol Biofuels. 2014. PMID: 24401161 Free PMC article.
-
Dehydrogenation Mechanism of Three Stereoisomers of Butane-2,3-Diol in Pseudomonas putida KT2440.Front Bioeng Biotechnol. 2021 Aug 26;9:728767. doi: 10.3389/fbioe.2021.728767. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34513815 Free PMC article.
-
Ketoreductase TpdE from Rhodococcus jostii TMP1: characterization and application in the synthesis of chiral alcohols.PeerJ. 2015 Nov 10;3:e1387. doi: 10.7717/peerj.1387. eCollection 2015. PeerJ. 2015. PMID: 26587349 Free PMC article.
-
Synthetic engineering of Corynebacterium crenatum to selectively produce acetoin or 2,3-butanediol by one step bioconversion method.Microb Cell Fact. 2019 Aug 6;18(1):128. doi: 10.1186/s12934-019-1183-0. Microb Cell Fact. 2019. PMID: 31387595 Free PMC article.
References
-
- Agranat I, Caner H, Caldwell J. Putting chirality to work: the strategy of chiral switches. Nat Rev Drug Discov. 2002;1:753–768. - PubMed
-
- Schoemaker HE, Mink D, Wubbolts MG. Dispelling the myths—biocatalysis in industrial synthesis. Science. 2003;299:1694–1697. - PubMed
-
- van der Donk WA, Zhao H. Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol. 2003;14:421–426. - PubMed
-
- Kim MJ, Whitesides GM. L-Lactate dehydrogenase: substrate specificity and use as a catalyst in the synthesis of homochiral 2-hydroxy acids. J Am Chem Soc. 1988;110:2959–2964. - PubMed
-
- Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, et al. Industrial biocatalysis today and tomorrow. Nature. 2001;409:258–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

