An electrostatically preferred lateral orientation of SNARE complex suggests novel mechanisms for driving membrane fusion
- PMID: 20126653
- PMCID: PMC2811192
- DOI: 10.1371/journal.pone.0008900
An electrostatically preferred lateral orientation of SNARE complex suggests novel mechanisms for driving membrane fusion
Abstract
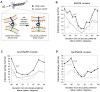
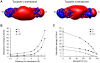
Biological membrane fusion is a basic cellular process catalyzed by SNARE proteins and additional auxiliary factors. Yet, the critical mechanistic details of SNARE-catalyzed membrane fusion are poorly understood, especially during rapid synaptic transmission. Here, we systematically assessed the electrostatic forces between SNARE complex, auxiliary proteins and fusing membranes by the nonlinear Poisson-Boltzmann equation using explicit models of membranes and proteins. We found that a previously unrecognized, structurally preferred and energetically highly favorable lateral orientation exists for the SNARE complex between fusing membranes. This preferred orientation immediately suggests a novel and simple synaptotagmin-dependent mechanistic trigger of membrane fusion. Moreover, electrostatic interactions between membranes, SNARE complex, and auxiliary proteins appear to orchestrate a series of membrane curvature events that set the stage for rapid synaptic vesicle fusion. Together, our electrostatic analyses of SNAREs and their regulatory factors suggest unexpected and potentially novel mechanisms for eukaryotic membrane fusion proteins.
Conflict of interest statement
Figures




References
-
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–9106. - PubMed
-
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. - PubMed
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Duman JG, Forte JG. What is the role of SNARE proteins in membrane fusion? Am J Physiol Cell Physiol. 2003;285:237–249. - PubMed
-
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

