Ret-PCP2 colocalizes with protein kinase C in a subset of primate ON cone bipolar cells
- PMID: 20127818
- PMCID: PMC2830016
- DOI: 10.1002/cne.22266
Ret-PCP2 colocalizes with protein kinase C in a subset of primate ON cone bipolar cells
Abstract
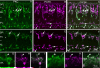
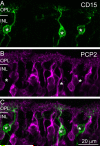
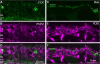
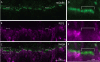
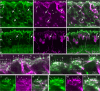
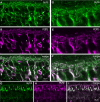
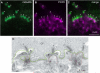
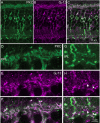
Purkinje cell protein 2 (PCP2), a member of the family of guanine dissociation inhibitors and a strong interactor with the G-protein subunit G alpha(o), localizes to retinal ON bipolar cells. The retina-specific splice variant of PCP2, Ret-PCP2, accelerates the light response of rod bipolar cells by modulating the mGluR6 transduction cascade. All ON cone bipolar cells express mGluR6 and G alpha(o), but only a subset expresses Ret-PCP2. Here we test the hypothesis that Ret-PCP2 contributes to shaping the various temporal bandwidths of ON cone bipolar cells in monkey retina. We found that the retinal splice variants in monkey and mouse are similar and longer than the cerebellar variants. Ret-PCP2 is strongly expressed by diffuse cone bipolar type 4 cells (DB4; marked with anti-PKCalpha) and weakly expressed by midget bipolar dendrites (labeled by antibodies against G alpha(o), G gamma 13, or mGluR6). Ret-PCP2 is absent from diffuse cone bipolar type 6 (DB6; marked with anti-CD15) and blue cone bipolar cells (marked with anti-CCK precursor). Thus, cone bipolar cells that terminate in stratum 3 of the inner plexiform layer (DB4) express more Ret-PCP2 than those that terminate in strata 3 + 4 (midget bipolar cells), and these in turn express more than those that terminate in stratum 5 (DB6 and blue cone bipolar cells). This expression pattern approximates the arborization of ganglion cells (GC) with different temporal bandwidths: parasol GCs stratifying near stratum 3 are faster than midget GCs stratifying in strata 3 + 4, and these are probably faster than the sluggish GCs that arborize in stratum 5.
(c) 2009 Wiley-Liss, Inc.
Figures


Similar articles
-
Connections of diffuse bipolar cells in primate retina are biased against S-cones.J Comp Neurol. 2007 May 1;502(1):126-40. doi: 10.1002/cne.21284. J Comp Neurol. 2007. PMID: 17335043
-
Expression of genes encoding glutamate receptors and transporters in rod and cone bipolar cells of the primate retina determined by single-cell polymerase chain reaction.Mol Vis. 2007 Nov 28;13:2194-208. Mol Vis. 2007. PMID: 18087239
-
Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response.J Neurosci. 2008 Sep 3;28(36):8873-84. doi: 10.1523/JNEUROSCI.0812-08.2008. J Neurosci. 2008. PMID: 18768681 Free PMC article.
-
The cone synapses of DB1 diffuse, DB6 diffuse and invaginating midget, bipolar cells of a primate retina.J Neurocytol. 1996 Jul;25(7):381-90. J Neurocytol. 1996. PMID: 8866239
-
Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina.Vis Neurosci. 2014 Mar;31(2):139-51. doi: 10.1017/S0952523813000230. Epub 2013 Jul 29. Vis Neurosci. 2014. PMID: 23895762 Free PMC article. Review.
Cited by
-
Differential function of Gγ13 in rod bipolar and ON cone bipolar cells.J Physiol. 2015 Apr 1;593(7):1531-50. doi: 10.1113/jphysiol.2014.281196. Epub 2015 Jan 2. J Physiol. 2015. PMID: 25416620 Free PMC article.
-
A systematic comparison of optogenetic approaches to visual restoration.Mol Ther Methods Clin Dev. 2022 Mar 7;25:111-123. doi: 10.1016/j.omtm.2022.03.003. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35402632 Free PMC article.
-
Cross-species single-cell landscapes identify the pathogenic gene characteristics of inherited retinal diseases.Front Genet. 2024 Jul 11;15:1409016. doi: 10.3389/fgene.2024.1409016. eCollection 2024. Front Genet. 2024. PMID: 39055259 Free PMC article.
-
Paraneoplastic CDR2 and CDR2L antibodies affect Purkinje cell calcium homeostasis.Acta Neuropathol. 2014 Dec;128(6):835-52. doi: 10.1007/s00401-014-1351-6. Epub 2014 Oct 24. Acta Neuropathol. 2014. PMID: 25341622 Free PMC article.
-
Immunocytochemical evidence that monkey rod bipolar cells use GABA.Eur J Neurosci. 2010 Feb;31(4):685-96. doi: 10.1111/j.1460-9568.2010.07106.x. Eur J Neurosci. 2010. PMID: 20384812 Free PMC article.
References
-
- Berrebi AS, Mugnaini E. Characteristics of labeling of the cerebellar Purkinje neuron by L7 antiserum. J Chem Neuroanat. 1992;5:235–243. - PubMed
-
- Berrebi AS, Oberdick J, Sangameswaran L, Christakos S, Morgan JI, Mugnaini E. Cerebellar Purkinje cell markers are expressed in retinal bipolar neurons. J Comp Neurol. 1991;308:630–649. - PubMed
-
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr., Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. - PubMed
-
- Chan TL, Martin PR, Grünert U. Immunocytochemical identification and analysis of the diffuse bipolar cell type DB6 in macaque monkey retina. Eur J Neurosci. 2001;13:829–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

