Improving the treatment of non-Hodgkin lymphoma with antibody-targeted radionuclides
- PMID: 20127947
- PMCID: PMC2820147
- DOI: 10.1002/cncr.24802
Improving the treatment of non-Hodgkin lymphoma with antibody-targeted radionuclides
Abstract
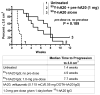
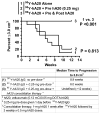
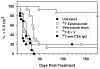
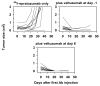
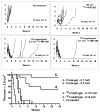
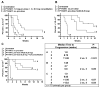
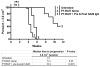
Radioimmunotherapy of non-Hodgkin lymphoma comprises a (90)Y- or (131)I-labeled murine anti-CD20 IgG, but both agents also include a substantial dose of unlabeled anti-CD20 IgG given immediately before the radioconjugate to reduce its uptake in the spleen (primary normal B-cell antigen sink); this extends its plasma half-life and improves tumor visualization. Thus, these treatments combine an effective anti-CD20 radioconjugate with an unconjugated anti-CD20 antibody that is also therapeutically active, but the large anti-CD20 IgG predose ( approximately 900 mg) may diminish the tumor localization of the radioimmunoconjugate (eg, 10-35 mg). We have examined alternative approaches that enhance radionuclide targeting and improve antitumor responses. One uses a (90)Y-labeled anti-CD22 IgG (epratuzumab) combined with an antibody therapy regimen of a humanized anti-CD20 IgG (veltuzumab). Pretargeted radionuclide therapy using a trivalent, humanized, recombinant bispecific anti-CD20 antibody with a (90)Y-hapten-peptide is another highly effective method that is also less toxic than directly radiolabeled IgG. Finally, all approaches benefit from the addition of a consolidation-dosing regimen of the anti-CD20 IgG antibody. This article reviews these various options and discusses how some fundamental changes could potentially enhance the response and duration from radionuclide-targeted therapy.
(c) 2010 American Cancer Society.
Figures

Similar articles
-
Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma.J Nucl Med. 2009 Mar;50(3):444-53. doi: 10.2967/jnumed.108.058602. Epub 2009 Feb 17. J Nucl Med. 2009. PMID: 19223402
-
Improved therapy of non-Hodgkin's lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody.Leukemia. 2005 Jun;19(6):1064-9. doi: 10.1038/sj.leu.2403751. Leukemia. 2005. PMID: 15815716
-
Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody.Cancer Res. 2008 Jul 1;68(13):5282-90. doi: 10.1158/0008-5472.CAN-08-0037. Cancer Res. 2008. PMID: 18593929 Free PMC article.
-
[Radioimmunotherapy for non-Hodgkin lymphoma: historical development and current status].Rev Esp Med Nucl. 2006 Jan-Feb;25(1):42-54. doi: 10.1157/13083351. Rev Esp Med Nucl. 2006. PMID: 16540013 Review. Spanish.
-
Administration guidelines for radioimmunotherapy of non-Hodgkin's lymphoma with (90)Y-labeled anti-CD20 monoclonal antibody.J Nucl Med. 2002 Feb;43(2):267-72. J Nucl Med. 2002. PMID: 11850494 Review.
Cited by
-
Poly(ADP-ribose) polymerase inhibitors combined with external beam and radioimmunotherapy to treat aggressive lymphoma.Nucl Med Commun. 2011 Nov;32(11):1046-51. doi: 10.1097/MNM.0b013e32834a369b. Nucl Med Commun. 2011. PMID: 21956491 Free PMC article.
-
In Vivo Pretargeted Imaging of HER2 and TAG-72 Expression Using the HaloTag Enzyme.Mol Pharm. 2017 Jul 3;14(7):2307-2313. doi: 10.1021/acs.molpharmaceut.7b00172. Epub 2017 Jun 8. Mol Pharm. 2017. PMID: 28505463 Free PMC article.
-
A systematic analysis of experimental immunotherapies on tumors differing in size and duration of growth.Oncoimmunology. 2012 Mar 1;1(2):172-178. doi: 10.4161/onci.1.2.18311. Oncoimmunology. 2012. PMID: 22720238 Free PMC article.
-
Radioimmunotherapy consolidation and rituximab maintenance in the initial treatment of follicular lymphoma.EJNMMI Res. 2011 Jun 20;1(1):7. doi: 10.1186/2191-219X-1-7. EJNMMI Res. 2011. PMID: 22214546 Free PMC article.
-
Radioimmunotherapy of non-Hodgkin's lymphoma: from the 'magic bullets' to 'radioactive magic bullets'.Yale J Biol Med. 2011 Dec;84(4):391-407. Yale J Biol Med. 2011. PMID: 22180677 Free PMC article. Review.
References
-
- Davies AJ. Radioimmunotherapy for B-cell lymphoma: Y90 ibritumomab tiuxetan and I131 tositumomab. Oncogene. 2007;26:3614–3628. - PubMed
-
- Buchegger F, Press OW, Delaloye AB, Ketterer N. Radiolabeled and native antibodies and the prospect of cure of follicular lymphoma. Oncologist. 2008;13:657–667. - PubMed
-
- Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–2463. - PubMed
-
- Davis TA, Kaminski MS, Leonard JP, et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10:7792–7798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

