Sporadic inclusion body myositis: variability in prevalence and phenotype and influence of the MHC
- PMID: 20128139
- PMCID: PMC2858953
Sporadic inclusion body myositis: variability in prevalence and phenotype and influence of the MHC
Abstract
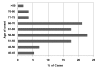
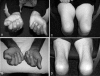
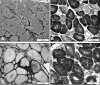
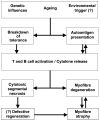
Sporadic inclusion body myositis (sIBM) is the most common myopathy presenting over the age of 40 years but its prevalence varies considerably in different populations. Genetic factors play a part in the pathogenesis of sIBM and in Caucasians susceptibility has been linked to the HLA-DR3 allele and the 8.1 MHC ancestral haplotype (AH) which is also associated with other autoimmune diseases. The variable prevalence of sIBM in different populations may be related to differences in the population frequency of this haplotype. Our recent observations indicate that the clinical phenotype at presentation is also quite variable and that the influence of the MHC is more complex than previously appreciated with HLA alleles also having modifying effects on the age-at-onset, severity and rate of progression of the disease. Recent recombinant mapping studies of polymorphisms in the Class II/III regions of the MHC by our group have further refined the susceptibility region and have identified a number of candidate genes warranting further investigation. The significance of these findings for the pathogenesis of the disease is discussed.
Figures




Similar articles
-
Two major histocompatibility complex haplotypes influence susceptibility to sporadic inclusion body myositis: critical evaluation of an association with HLA-DR3.Tissue Antigens. 2004 Nov;64(5):575-80. doi: 10.1111/j.1399-0039.2004.00310.x. Tissue Antigens. 2004. PMID: 15496200
-
The association of sporadic inclusion body myositis and Sjögren's syndrome in carriers of HLA-DR3 and the 8.1 MHC ancestral haplotype.Clin Neurol Neurosurg. 2011 Sep;113(7):559-63. doi: 10.1016/j.clineuro.2011.03.016. Epub 2011 Apr 20. Clin Neurol Neurosurg. 2011. PMID: 21507567
-
Recombination mapping of the susceptibility region for sporadic inclusion body myositis within the major histocompatibility complex.J Neuroimmunol. 2011 Jun;235(1-2):77-83. doi: 10.1016/j.jneuroim.2011.02.011. Epub 2011 May 2. J Neuroimmunol. 2011. PMID: 21543121
-
Genetics of inclusion-body myositis.Muscle Nerve. 2007 May;35(5):549-61. doi: 10.1002/mus.20766. Muscle Nerve. 2007. PMID: 17366591 Review.
-
Genetic advances in sporadic inclusion body myositis.Curr Opin Rheumatol. 2015 Nov;27(6):586-94. doi: 10.1097/BOR.0000000000000213. Curr Opin Rheumatol. 2015. PMID: 26335925 Review.
Cited by
-
The Clinical and Histological Spectrum of Idiopathic Inflammatory Myopathies.Clin Rev Allergy Immunol. 2017 Feb;52(1):88-98. doi: 10.1007/s12016-015-8517-4. Clin Rev Allergy Immunol. 2017. PMID: 26514357 Review.
-
A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy.Cells. 2019 Jul 3;8(7):674. doi: 10.3390/cells8070674. Cells. 2019. PMID: 31277291 Free PMC article. Review.
-
Idiopathic Inflammatory Myopathies: an Update on Classification and Treatment with Special Focus on Juvenile Forms.Clin Rev Allergy Immunol. 2017 Feb;52(1):34-44. doi: 10.1007/s12016-015-8512-9. Clin Rev Allergy Immunol. 2017. PMID: 26429707 Review.
-
Inflammasome in Skeletal Muscle: NLRP3 Is an Inflammatory Cell Stress Component in Inclusion Body Myositis.Int J Mol Sci. 2023 Jun 26;24(13):10675. doi: 10.3390/ijms241310675. Int J Mol Sci. 2023. PMID: 37445853 Free PMC article.
-
Inclusion body myositis: therapeutic approaches.Degener Neurol Neuromuscul Dis. 2012 May 10;2:43-52. doi: 10.2147/DNND.S19899. eCollection 2012. Degener Neurol Neuromuscul Dis. 2012. PMID: 30890877 Free PMC article. Review.
References
-
- Needham M, Corbett A, Day T, et al. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci 2008;15:1350-3. - PubMed
-
- Khadilkar SV, Patil SG, Amin SN. Study of idiopathic inflammatory myopathies with special reference to borderland between idiopathic inflammatory myopathies and muscular dystrophies. Neurol India 2008;56:356-62. - PubMed
-
- Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol 1995;38:705-13. - PubMed
-
- Needham M, James I, Corbett A, Mastaglia, FL, et al. Sporadic inclusion body myositis: phenotypic variability and influence of HLA-DR3 in a cohort of 57 Australian cases. J Neurol Neurosurg Psychiatry 2008;79:1056-60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
