Oxaliplatin responses in colorectal cancer cells are modulated by CHK2 kinase inhibitors
- PMID: 20128802
- PMCID: PMC2848936
- DOI: 10.1111/j.1476-5381.2009.00607.x
Oxaliplatin responses in colorectal cancer cells are modulated by CHK2 kinase inhibitors
Abstract
Background and purpose: Checkpoint kinase 2 (CHK2) is activated by DNA damage and can contribute to p53 stabilization, modulating growth arrest and/or apoptosis. We investigated the contribution of CHK2 to oxaliplatin-mediated toxicity in a colorectal cancer model.
Experimental approach: We evaluated the ability of CHK2 small molecule inhibitors to potentiate oxaliplatin-induced toxicity. The role of CHK2 in oxaliplatin-induced apoptosis was investigated in HCT116 cells that were wild-type (WT) or KO for CHK2. Small molecule inhibitors of CHK2 were used in combination studies with oxaliplatin in this cell model.
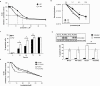
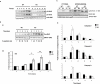
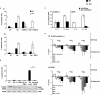
Key results: In oxaliplatin-treated CHK2 KO cells, accelerated apoptosis was accompanied by attenuated p53 stabilization and p21(WAF-1) up-regulation correlating with increased Bax expression, cytochrome c release and elevated caspase activity. The higher levels of apoptosis in CHK2 KO cells were restored to control (WT) levels when CHK2 was re-introduced. This 'uncoupling' of p53 stabilization and Bax up-regulation in CHK2 KO cells suggested oxaliplatin-induced apoptosis was due to a p53-independent response. Combination studies revealed that CHK2 inhibitor II or debromohymenialdisine antagonized the responses to oxaliplatin. This inhibitory effect correlated with decreases in apoptosis, p53 stabilization and DNA inter-strand cross-link formation, and was dependent on the presence (but not activity) of CHK2.
Conclusions and implications: Combinations of CHK2 inhibitors with oxaliplatin should further sensitize cells to oxaliplatin treatment. However, these inhibitors produced an antagonistic effect on the response to oxaliplatin, which was reversed on the re-introduction of CHK2. These observations may have implications for the use of oxaliplatin in colorectal cancer therapy in combination with therapies targeting CHK2.
Figures

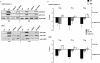
Similar articles
-
Enhanced oxaliplatin-induced apoptosis following antisense Bcl-xl down-regulation is p53 and Bax dependent: Genetic evidence for specificity of the antisense effect.Mol Cancer Ther. 2004 Feb;3(2):169-78. Mol Cancer Ther. 2004. PMID: 14985457
-
UCN-01 inhibits p53 up-regulation and abrogates gamma-radiation-induced G(2)-M checkpoint independently of p53 by targeting both of the checkpoint kinases, Chk2 and Chk1.Cancer Res. 2002 Oct 15;62(20):5743-8. Cancer Res. 2002. PMID: 12384533
-
Oxaliplatin-induced gamma-H2AX activation via both p53-dependent and -independent pathways but is not associated with cell cycle arrest in human colorectal cancer cells.Chem Biol Interact. 2009 Dec 10;182(2-3):173-82. doi: 10.1016/j.cbi.2009.08.019. Epub 2009 Sep 6. Chem Biol Interact. 2009. PMID: 19735649
-
Targeting chk2 kinase: molecular interaction maps and therapeutic rationale.Curr Pharm Des. 2005;11(22):2855-72. doi: 10.2174/1381612054546716. Curr Pharm Des. 2005. PMID: 16101442 Review.
-
Investigation into the Neuroprotective and Therapeutic Potential of Plant-Derived Chk2 Inhibitors.Int J Mol Sci. 2024 Jul 15;25(14):7725. doi: 10.3390/ijms25147725. Int J Mol Sci. 2024. PMID: 39062967 Free PMC article. Review.
Cited by
-
Single-Cell Transcriptomics Reveals Heterogeneity and Drug Response of Human Colorectal Cancer Organoids.Annu Int Conf IEEE Eng Med Biol Soc. 2018 Jul;2018:2378-2381. doi: 10.1109/EMBC.2018.8512784. Annu Int Conf IEEE Eng Med Biol Soc. 2018. PMID: 30440885 Free PMC article.
-
LncRNA LIMp27 Regulates the DNA Damage Response through p27 in p53-Defective Cancer Cells.Adv Sci (Weinh). 2023 Mar;10(7):e2204599. doi: 10.1002/advs.202204599. Epub 2023 Jan 13. Adv Sci (Weinh). 2023. PMID: 36638271 Free PMC article.
-
Distinct gene expression signatures in lynch syndrome and familial colorectal cancer type x.PLoS One. 2013 Aug 12;8(8):e71755. doi: 10.1371/journal.pone.0071755. eCollection 2013. PLoS One. 2013. PMID: 23951239 Free PMC article.
-
A confidence building exercise in data and identifiability: Modeling cancer chemotherapy as a case study.J Theor Biol. 2017 Oct 27;431:63-78. doi: 10.1016/j.jtbi.2017.07.018. Epub 2017 Jul 19. J Theor Biol. 2017. PMID: 28733187 Free PMC article.
-
Marine Sponge Natural Products with Anticancer Potential: An Updated Review.Mar Drugs. 2017 Oct 13;15(10):310. doi: 10.3390/md15100310. Mar Drugs. 2017. PMID: 29027954 Free PMC article. Review.
References
-
- Ahn J, Urist M, Prives C. Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J Biol Chem. 2003;278(23):20480–20489. - PubMed
-
- Almeida GM, Duarte TL, Steward WP, Jones GD. Detection of oxaliplatin-induced DNA crosslinks in vitro and in cancer patients using the alkaline comet assay. DNA Repair (Amst) 2006;5(2):219–225. - PubMed
-
- Antoni L, Sodha N, Collins I, Garrett MD. CHK2 kinase: cancer susceptibility and cancer therapy – two sides of the same coin? Nat Rev Cancer. 2007;7(12):925–936. - PubMed
-
- Arienti KL, Brunmark A, Axe FU, McClure K, Lee A, Blevitt J, et al. Checkpoint kinase inhibitors: SAR and radioprotective properties of a series of 2-arylbenzimidazoles. J Med Chem. 2005;48(6):1873–1885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

