Delta-aminolevulinic acid is a substrate for the amino acid transporter SLC36A1 (hPAT1)
- PMID: 20128809
- PMCID: PMC2848937
- DOI: 10.1111/j.1476-5381.2009.00620.x
Delta-aminolevulinic acid is a substrate for the amino acid transporter SLC36A1 (hPAT1)
Abstract
Background and purpose: delta-Aminolevulinic acid (ALA) is used in cancer patients for photodynamic diagnosis or therapy. Oral administration of ALA has been used in patients with prostate and bladder cancer. The present aim was to investigate the mechanism of intestinal absorption of ALA and its transport via the amino acid transporter SLC36A1.
Experimental approach: In vitro investigations of ALA affinity for and uptake via SLC36A1 and SLC15A1 were performed in Caco-2 cell monolayers. Interaction of ALA with SLC15A1 was investigated in MDCK/SLC15A1 cells, whereas interactions with SLC36A1 were investigated in COS-7 cells transiently expressing SLC36A1.
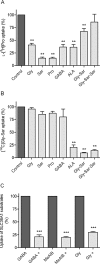
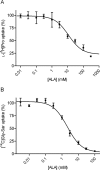
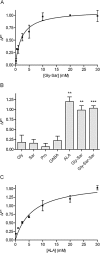
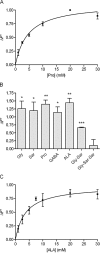
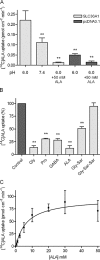
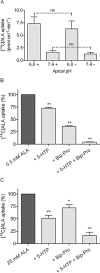
Key results: ALA inhibited SLC36A1-mediated L-[(3)H]Pro and SLC15A1-mediated [(14)C]Gly-Sar uptake in Caco-2 cell monolayers with IC(50) values of 11.3 and 2.1 mM respectively. In SLC36A1-expressing COS-7 cells, the uptake of [(14)C]ALA was saturable with a K(m) value of 6.8 +/- 3.0 mM and a V(max) of 96 +/- 13 pmol x cm(-2) x min(-1). Uptake of [(14)C]ALA was pH and concentration dependent, and could be inhibited by glycine, proline and GABA. In a membrane potential assay, translocation of ALA via SLC36A1 was concentration dependent, with a K(m) value of 3.8 +/- 1.0 mM. ALA is thus a substrate for SLC36A1. In Caco-2 cells, apical [(14)C]ALA uptake was pH dependent, but Na(+) independent, and completely inhibited by 5-hydroxy-L-tryptophan and L-4,4'-biphenylalanyl-l-proline. CONCLUSIONS AND IMPLICATIONS. ALA was a substrate for SLC36A1, and the apical absorption in Caco-2 cell was only mediated by SLC36A1 and SLC15A1. This advances our understanding of intestinal absorption mechanisms of ALA, as well as its potential for drug interactions.
Figures
References
-
- Addison JM, Burston D, Matthews DM. Evidence for active transport of the dipeptide glycylsarcosine by hamster jejunum in vitro. Clin Sci. 1972;43:907–911. - PubMed
-
- Andersen R, Nielsen CU, Begtrup M, Jorgensen FS, Brodin B, Frokjaer S, et al. In vitro evaluation of N-methyl amide tripeptidomimetics as substrates for the human intestinal di-/tri-peptide transporter hPEPT1. Eur J Pharm Sci. 2006;28:325–335. - PubMed
-
- Anderson CM, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, et al. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

